EU MDR Requirements for Implant Cards
Information for Manufacturers of Implantable Medical Devices
According to Article 18 of Regulation (EU) 2017/745 on medical devices, the European Union (EU) requires manufacturers to provide “implant cards” for patients with implanted medical devices. The intention of the requirement is to make crucial information about implants easily accessible to patients. Device manufacturers are responsible for manufacturing the implant cards according to specific formatting, information, and language requirements. Providers or medical institutions are responsible for delivering the cards to patients.
Implantable Devices
The MDR definition of an implantable device is:
‘implantable device’ means any device, including those that are partially or wholly absorbed, which is intended:
- to be totally introduced into the human body, or
- to replace an epithelial surface or the surface of the eye,
by clinical intervention and which is intended to remain in place after the procedure.
Any device intended to be partially introduced into the human body by clinical intervention and intended to remain in place after the procedure for at least 30 days shall also be deemed to be an implantable device.
It is also important to match your implantable device with the correct classification.
How Implant Cards improve patient safety
The implant card must be wallet-sized and specific to the implanted medical device. They are created in three ways to improve patient safety:
- Through accurate information: Patients can more readily get information on a device by identifying the unique implant in their body, including name, serial number, and lot number. They can also access EUDAMED, the European database on medical devices, and other websites.
- By means of practical identification: Patients can demonstrate that they require extra attention, for example, at airport security checkpoints.
3. During emergency care: In the case of a medical emergency, the cards can let emergency doctors and first responders know if a patient has an implanted device.
Implant Card guidelines
The Medical Device Coordination Group (MDCG) document – MDCG 2021-11 Guidance on Implant Card – ‘Device Types’ provides a non-exhaustive list of medical devices that come under the implant card regulation.
MDCG 2019-8 also sets out very specific instructions for how the implant card should look and what information it should contain. It also gives very clear examples and explanations for this.
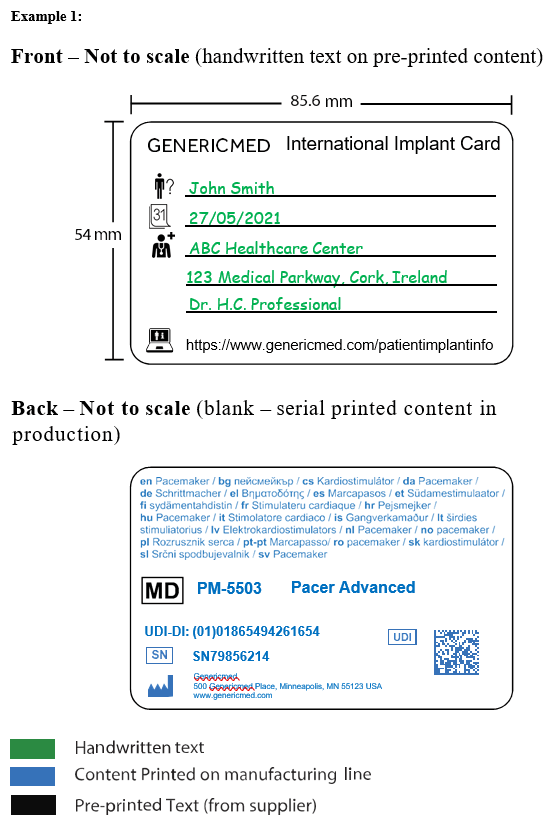
The implant cards must be the size of a credit card, ATM card or ID card (wallet-sized). All text (letter, numbers, and symbols) must be at least 2 millimetres high and easily understood by laypersons. Information must also be in the language determined by the relevant member state.
The card should list the following information about the implant:
- Device name
- Device type
- Serial number (or lot or batch number)
- Unique Device Identification (UDI)
- Name, address, and website of manufacturer
The UDI should be in automatic identification and data capture format, such as linear or 2D-barcodes. The UDI device identifier (UDI-DI) should be in human-readable format.
The card also must include blank fields. These are for patient identification information and will be filled out by the healthcare institution or provider. This information gives:
- Patient name or ID
- Implantation date
- Name and address of the healthcare institution or provider who performed the implantation.
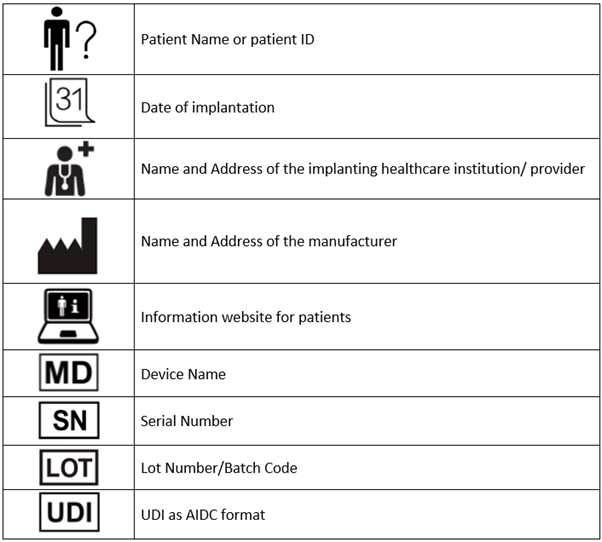
It is also advisable to use internationally recognisable symbols on the implant card. The MDCG guidance provides this as per the table below:
Some suggested designs are also included in the MDCG guidance, such as:
Key information - EU MDR Article 18 requirements
- Implant cards don’t need to be provided retrospectively. The requirements only apply to devices placed on the market under EU MDR 2017/245.
- Manufacturers are required to have a website to communicate with patients of implants.
- The manufacturer must demonstrate compliance with Article 18 and justify this.
- Patients should also be provided with an information leaflet to accompany their implant card.
- All required information must be present on both the implant card and the manufacturers website.
- All implants apart from those on thew exemption list in Article 18 must have an implant card.
- The implant card must be human readable, and the UDI-DI and UDI-PI must be included.
Clin-r+ recommendations
It is important for manufacturers of implantable devices to understand what the requirements are under EU MDR and how they affect their device(s).
In order to be compliant, manufacturers need to understand device classification, requirements, conformity assessment, clinical evaluation and investigation, post market surveillance, traceability, and instructions for use.
There is a lot of guidance available, but it can still be a minefield to get it right. Clin-r+ has a wealth of experience to call upon. We can assist you with all the recommendations above to ensure you’re MDR compliant. To learn more about our services and how we can help, get in touch!

