Orphan Medical Devices
Information for Manufacturers
Orphan medical devices are products or equipment intended for the prediction, prevention, diagnosis, support, treatment and management of life-threatening or chronically debilitating diseases with a low prevalence/incidence. In many countries worldwide orphan drug regulations are installed but only the United States of America and Japan have an orphan device regulation. For many years surgeons have used off-label or self-assembled medical devices for the prevention, diagnosis or treatment of rare disorders. The EU MDR’s regulations on clinical data ensures that the medical device being used on the general public for their treatment or diagnosis is safe, but there is no orphan pathway.

What are Orphan Medical Devices
Orphan medical devices are products or equipment intended for patient populations with a low prevalence or incidence. These diseases are potentially life-threatening or life-altering leading to long-term disability. Orphan medical technology is both the device and equipment that connects to other devices.
The term ‘orphan’ is due the lack of interest and support that these innovations suffer. Orphan medical devices have high costs to develop and test. But often has low return on investment due to the small community it benefits, which means that they aren’t as attractive to stakeholders for funding development.
Orphan Medical Devices and Medicinal Products
The Medical Device Coordination Group (MDCG) has acknowledged the need to define ‘orphan’ status to certain devices. Currently work is being undertaken to provide manufacturers with a definition for orphan devices and the formulation for specific guidance or other means of assistance for those products to be able to meet the legal requirements. However, no regulatory framework is currently available.
The MDCG also recognises that sustainable solutions are needed in the mid- and long-term for orphan devices.
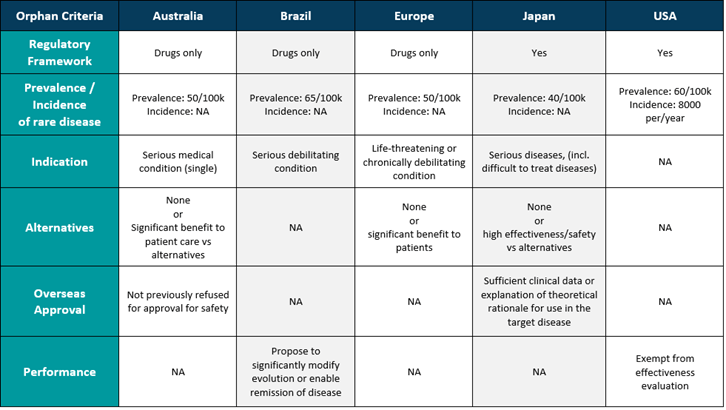
The European Commission give information on orphan medical products and provided a criteria to qualify for orphan designation in medicine. So, it is highly likely that the MDCG guidance, when released, will follow a similar framework for medical devices.
Orphan medical devices are already regulated in countries such as the USA and Japan. The table below shows an overview of whether orphan medical devices are included.
Orphan Medical Devices and EU MDR
Due to the increased requirements for clinical data for CE marking, the MDR may inadvertently make it harder to secure orphan medical devices. As a result, innovation for these conditions will be stunted. For rare conditions, clinical trials are complex due to the limited population and complexity to obtain real world data. Observational study articles and post-market surveillance data are often the only real-world evidence that these devices work.
Passive post-market surveillance by manufacturers could be limited and clinical data underreported, so it wouldn’t be enough on its own. This means that more clinical data needs to be gathered through clinical investigations and possibly active post-market surveillance. This may be the case even after manufacturers have put in a lot of time and money to do this.
The MDR doesn’t currently make a specific exception for orphan medical devices. However, it was addressed in the opening remarks of the Commissioner at the EPSCO Council in December 2022. “As a priority to develop solutions on orphan devices. These initiatives have been started to ensure patients with rare diseases continue to have access to these innovations. Together with the dedicated Medical Device Coordination Group taskforce the European commission have committed to identify medium and long term solutions.”
The Commission also published a factsheet for supporting the transition to the new medical device framework which mentions orphan devices as being crucial and needing tailored solutions in the longer term.
Currently without a regulatory framework for orphan devices, it is not clear how to overcome the orphan challenge for CE certification. However, there may be other Articles within the EU MDR that could provide some support for current submissions:
Article 59
Article 59 Derogation from the conformity assessment procedures could be applicable. Even though it wasn’t made for orphan medical devices, Article 59 of the MDR says that a waiver can be made for devices that don’t have the CE mark. With this exception, an approval from a national competent authority can be used instead of the normal conformity assessment by notified bodies. This can be done for the health and safety of the public or of the patient. Article 59 can only be used in certain situations because it requires multiple approvals at the national level. This adds time and money to the process of making these devices. Also, this exception is only valid for a certain amount of time.
Article 97
Orphan medical devices that don’t follow the MDR could be dealt with on a case-by-case basis, as Article 97 of the MDR. But again, this approach can only be used for a limited time because the competent authorities are required to give a reasonable amount of time to resolve the non-compliance. Therefore, this makes the strategy unsustainable.
Benefit-Risk
Due to the lack of regulatory framework non-sustainable ways to market an orphan device, we suggest justifying the critical clinical need the device solves in the marketplace. This needs to be based on the benefit-risk profile and the current state of the art for the treatment/diagnosis of the medical condition the device is intended for.
Demonstrating the device’s clinical need, the negative impact if the need is not address can provide a benefit-to-risk ratio in the absence of statistically significant clinical data. The negative impact of no treatment, the clinical benefits the device provides and the fact that there are no alternatives must compensate for the lack of clinical data. So even if the device doesn’t have enough clinical data, the fact that there isn’t another way to treat or diagnose a life threatening or serious/chronic debilitating condition can be used to justify the benefits it offers to patients.
The benefit-risk profile will only be considered acceptable if robust PMCF activities are in place. These must prove the validity of the benefit-risk and continue to collect further clinical data in order to attain a statistically significant level of evidence.
Clin-r+ recommendations
Whilst there are no specific guidelines for EU MDR, the strategy for each possible orphan medical device will be different and will rely on a number of things. The quality of the clinical evidence available and how the device affects health are prime examples of what needs to be considered.
It would also be beneficial for manufacturers of orphan medical devices to familiarise themselves with Regulation (EC) No 141/2000 on orphan medicinal products. This provides criteria for the designation of medicinal products.
CLIN-r+ can assist and support manufacturers identify orphan medical devices and the best course of action to take for regulatory compliance. We can provide professional assistance and clarify any issues. CLIN-r+ has a wealth of experience to call upon. Get in touch!

