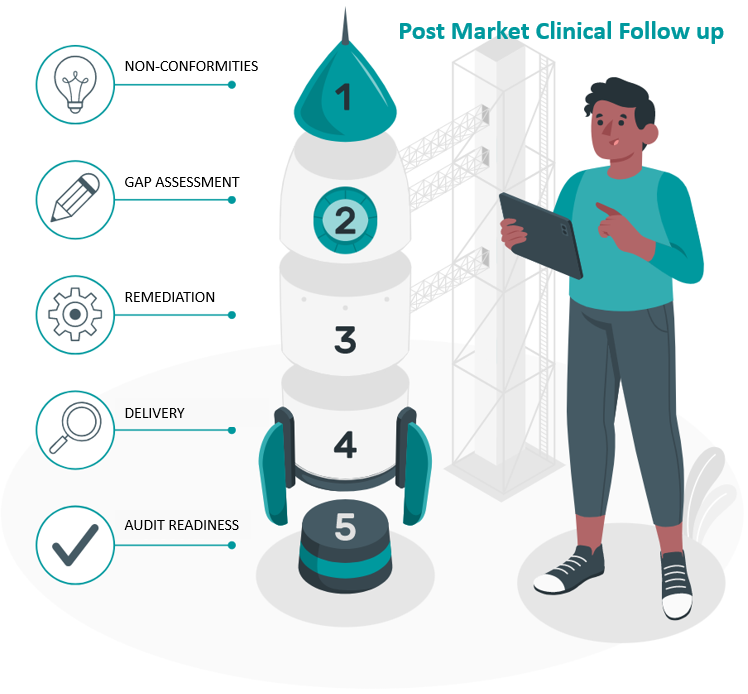
Remediating biocompatibility workflows to meet EU MDR compliance requirements
This case study looks at remediating biocompatibility workflows to meet EU MDR compliance requirements. CLIN-r+ supported the manufacturer with their problematic biocompatibility workflows to address audit findings and deficiencies and pass the second NB assessment.