CLIN-r+ unequalled knowledge
CLIN- r+ is a specialist Medical Affairs, Regulatory and Assurance consultancy in Medical Devices and In Vitro Diagnostics. One of our greatest strengths is our team’s unequalled knowledge, which we offer to your project. When selecting a clinical affairs, assurance and regulatory partner, one of the most crucial elements to consider is evolving expertise and strategic edge.
CLINr+ Services
At CLIN-r+ we’re a trusted Clinical Regulatory affairs partner to manufacturers of medical and in-vitro diagnostics devices manufacturers worldwide. We ensure our clients remain in compliance with regulatory standards. Our clinical, IVD, and technical documentation services provide audit and inspection readiness

Regulatory Affairs Management

SLR for SoTA, Safety and Performance Data
Clinical Evaluation Reports (CER)

PMS and QMS Remediation
Clinical and Technical Documentation
PMCF Plans and SAP
Validation Project Management
Quality and Validation
Case Studies
Have a look at our case studies how we have helped manufacturers tame and master their regulatory compliance:
WHAT OUR CLIENTS SAY:
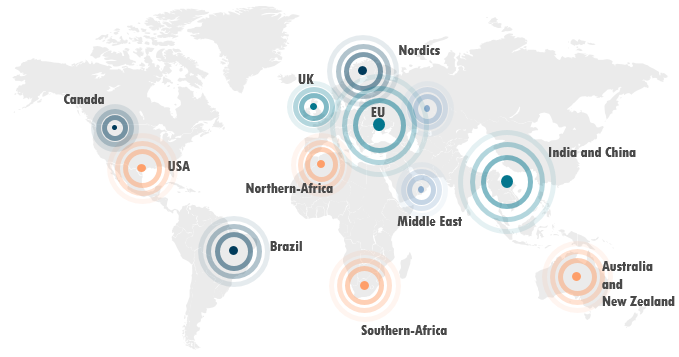
We are honored to have clients worldwide
Why CLINr+?
CLIN-r+ is a specialist Medical Device consultancy of Medical, Regulatory, QA Engineering and Research professionals who specialise in creating compliant Technical Documents, SOP’s and CERs. Our Clinical Affairs teams consist of medical professionals and seasoned Medical Device developers so it comes as no surprise that our improved clinical evaluation process has a track record of audit success. For medical devices of all classes, we create and maintain compliance with Clinical Evaluation Reports and associated paperwork. To ensure rapid success, we bring medical key opinion leaders, researchers, medical writers, regulatory consultants, toxicologists and biostatisticians to your team to supplement the know-how of your existing teams. Collectively we have over 20 years of technical writing experience helping medical device manufacturers build CERs, support MDD to MDR migrations and Health Technology assessments with verified success.
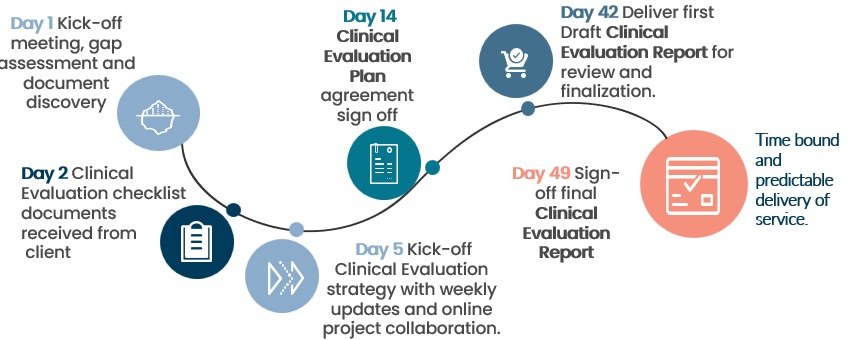
So it comes as no surprise that our first draft of the CER can be delivered within 6 weeks* to our clients.
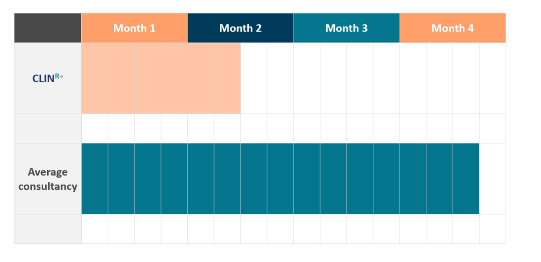
CLINR+ utilizes experts in regulatory, systematic review researchers and medical writers, we invest in systematic review software and NICE validated tools to provide a clear workflow for our clients. The service is fast tracked as collaboration of key inputs is done real time online with clients ensuring swift progress. Flexibility and advisory support is also provided in the case of gaps.
Our Affiliations:














