Safety & Performance Clinical Data (trials and literature)
What is Safety and Performance Clinical Data?
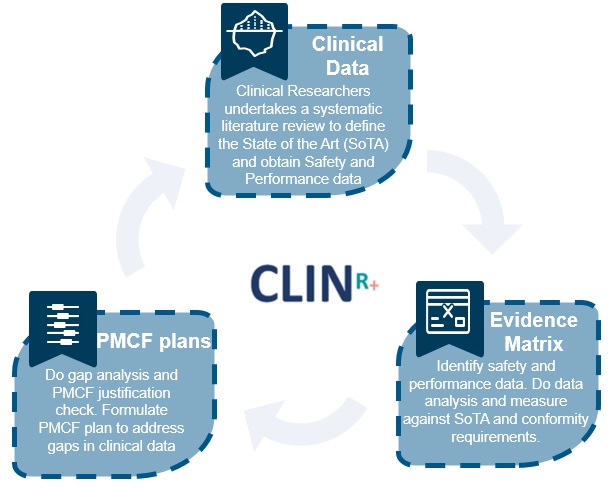
All medical devices must have adequate clinical evidence to demonstrate compliance with relevant, essential safety and performance requirements. Undertaking a Literature Search establishes the safety and performance of the device, and also includes a review of the current knowledge and/or state of the art (SOTA). These two sections work together to help the device synthesis improve its clinical safety and performance.
What do you need to be compliant?
Undertaking a SOTA literature review is much more cost effective than planning or executing a Clinical Trial before anything else. This will ensure you define the performance and safety range for your device category and alternative therapies. Having this benchmark will also ensure you clearly show that you meet the GSPRs and establish if you have adequate data. It also identifies if data is missing and what PMCF studies you need to undertake.
How can Clin-r+ help?
CLIN-r+ has a team of industry expert reviewers and medical writers. Using proven methodologies, we collect unbiased, independent assessment data x6 faster through Clinical Experience Activities that feeds into your regulatory submission. We provide resources such as clinical study designers, statisticians and experienced SOTA medical writers to systematically review data and help expand our clients’ capabilities.
The CLIN-r+ team prides themselves on using clearly defined protocols which are effectively planned before conducting searches. They are exceedingly thorough, consistent and cover a wide range of parameters, objectively documenting the findings in detail and including good, bad, and justifiable results to give you everything needed to move forward.
Where limited data from the manufacturer is available, we find clinical data from scientific literature of equivalent/similar products that can be a substantial portion of clinical evidence. Then we only include the assessed clinical data set identified in the clinical evaluation following a series of thorough tests to ensure its suitability.
Need more details?
We are here to assist. Contact us, set up a meeting, join our mailing list or follow us on LinkedIn.
You can also check out these white papers on our MedTech Academy:
- Clinical Investigations for Medical Devices and IVD
- Clinical Development Plan (CDP)
- State Of The Art Literature Reviews
- Performing Effective Literature Searches
- Three Tips for PMCF Planning
