Sustainability Assessments – Future Impact on Medical Device QMS
Evolving the QMS Design Process, Validations, Risk Management and Clinical Evaluation
In future meeting all local and global sustainability regulations will be a must for all companies doing business in the 21st century. For MedTech manufacturers this new area of conformance will have a profound impact on device design controls (specifically material validation and processing validations), risk management process (ISO 14971) and clinical evaluation justification. Early adapters to this change are generally surprised by the impact on the clinical evaluation process, but devastating impact of climate change on human health becomes clearer, (WHO) it’s undeniable that it will be factored into the benefit -risk profile. The WHO vision for an environmentally sustainable health system is one that improves, maintains, or restores health, while minimizing negative impacts on the environment and leveraging opportunities to restore and improve it, to the benefit of the health and well-being of current and future generations. Hence the lifecycle impact of your medical device will extend beyond its use and include its disposal and potential reuse.
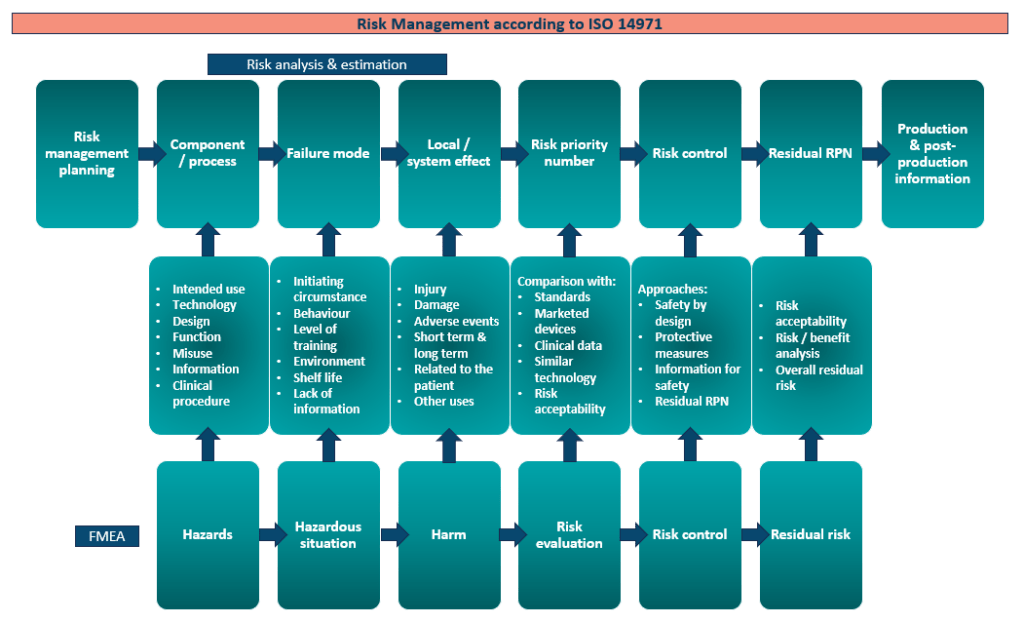
Outlined in the current risk management process for manufacturers which shows an absence of sustainability assessments. Include incorporating the environmental impact of your medical device and your business practices will soon either e another harmonised standard on an update in your exciting QMS or ISO 14971. Being proactive to this change is imperative as if ignored it will undermine the clinical benefits that your product brings to the marketplace. You will need to assess the overall benefit that the product provides for its intended purpose against the risks (device harms, environmental harms, and social harms) involved. The benefit-risk profile of the device is a deciding factor the EU MDR certification but also recently for market access as procurement offices are asking how sustainable is your medical device?
What effect does this have on your clinical assessment?
The social and environmental determinants of health, such as clean air, safe drinking water, enough food, and adequate shelter, are impacted by climate change. Between 2030 and 2050, it is predicted that climate change will result in an additional 250 000 fatalities year from malnutrition, malaria, diarrhoea, and heat stress.
The risk assessment will take into account your device’s environmental impact. If there is zero-impact, it won’t undermine the benefits of your device. However, if the environmental impact of producing your device exceeds the benefit it poses to the healthcare sector it may become redundant.
Organization such as WHO are proposing ten avenues with initiates such as NHS Net Zero quickly being initiated. These changes will be rapid. Guidelines and ISO standards are being formulated on how the environmental impact should be assessed for medical devices. But currently we are using cross over guidance from other industries and their applicable ISO standards to outline the way forward. The initial carbon footprint methodology has evolved towards a more comprehensive ‘Material Life Cycle’ analysis and ‘Circular Economy strategy’ which is earmarked to be the most suitable methodology to assess environmental impact for MedTech devices.
What is lifecycle analysis?
A life cycle assessment (LCA) is a methodology for assessing environmental impacts associated with all the stages of the life cycle of a medical device. A nationally approved method of assessment must be used to conduct an LCA. LCA takes into consideration all the steps that lead from raw material through manufacture, distribution, and usage to final disposal. There must be evidence of emissions reduction measures. Operational, embodied, and post “end of life” benefits must all be considered in the assessment.
Stages in the life of a project that must be provided over the life cycle of a medical device:
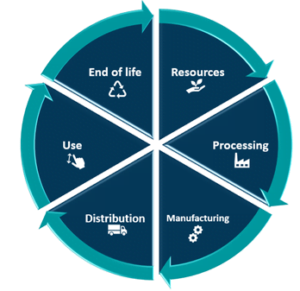
- Material compliance – Materials compliance is set to become a big buzz word for your Design SOP and critical component in your Design History File (DHF). To cut carbon emissions at this stage as well as the stages that follow, consider how your products are sourced, carbon footprint validation and made.
- Device production – The production process and materials used in production will need to comply with health, safety, and environmental regulations surveyed by authorities such as the EPA, OSHA, and ECHA ext. The processes used in product fabrication and construction will require further verification and validation work for the production teams. And would require support from regulatory department to ensure all compliance standards are being considered.
- Use – Understanding the device’s capabilities will help you reduce future emissions from maintenance, replacement, and repair work as well as operational energy use by properly taking into account the device’s total resource efficiency.
- End of life – Until the device lifecycle is over, and its parts are ready for future use, collect emissions from deconstruction and recycling, transport, reprocessing, reuse, and disposal.
Benefits and loads beyond the system boundary – Create scenarios for what will happen to a device when it is disassembled to assist in future reuse, reprocessing, or recycling. The circular economy module is made up of this and the preceding module.
What is material and substance management?
Material and substance management is a key area for any company that builds physical products. Expect your validation process to evolve beyond your currently required product lifetime to include validations for reprocessing and disposal. It is important to explore materials based on the three types to understand it’s impact on validations:
- Production materials – used to make medical equipment. (note that some production materials may not end up in final products; they include, for example, volatile organic compounds that evaporate within a period of time)
- Non-production materials – they are used in the production process to run the facility, but do not end up in production parts, e.g., conveyor belts used at plants to produce parts, toilet paper used in facilities, fuel in delivery vehicles, paper to print the IFU, labelling and packaging boxes to ship physical parts –, and
- Post-production materials – including service materials and chemicals. Each type of material includes two major categories: dimensional materials – i.e. articles with shape, hard parts, e.g., medical needles, rubber balls, and steels – and non-dimensional materials – i.e. wet chemicals, mixtures, and fluids.
Production, non-production, and post-production materials and substances are often managed in different ways, resulting in multiple processes across multiple departments and business units. In many cases, these processes have evolved separately, at different times, with different objectives in mind, and often using different IT tools and databases. Material and substance management is quite a complex task spanning across the business and considering its direct impact on company operations and the bottom line, should be tackled in readiness for regulatory changes.

What ISO’s can be used?
There is no telling at this stage whether the risk management and the quality management ISOs for medical devices will be updated with sustainability guidance or ISO standards that are currently in circulation. But it is safe to safe its worthwhile for manufacturers to start familiarising and strategically planning around the guidance that is available. Below we will quickly discuss the main sustainability ISOs that should be considered at this stage.
ISO 14001 Environmental Standard
Although ISO 14001 certification is not mandatory, it could increase medical device company competitiveness and contribute to saving the environment. By lowering environmental impact while promoting sustainable practices, the ISO 14001 Environmental Management System (EMS) is essential to the medical sector and medical device manufacture. By carefully managing environmental aspects and repercussions, this standard ensures compliance with environmental regulations and strengthens corporate social responsibility (CSR).
A structured system like ISO 14001 enables an organisation to meet regulatory requirements, as well as its financial and business goals, while also achieving its stated environmental objectives. Although ISO 14001 and ISO 9000 are related to quality systems (ISO QMS STANDARD), ISO 14001 is far more user-friendly.
According to ISO 14001, an environmental management system should be “part of an overall management system that includes organisational structure, planning activities, responsibilities, practises, procedures, processes, and resources for developing, implementing, achieving, reviewing, and maintaining environmental policy.” This standard includes the components that are common to all successful management systems.
To comply with ISO 14001, you must first identify and then manage all significant environmental aspects of its activities, products, or services. Once the processes that have an impact on the environment have been identified, you can create the objectives, targets, and programmes required to manage those effects, achieve objectives, and comply with all other applicable regulations.
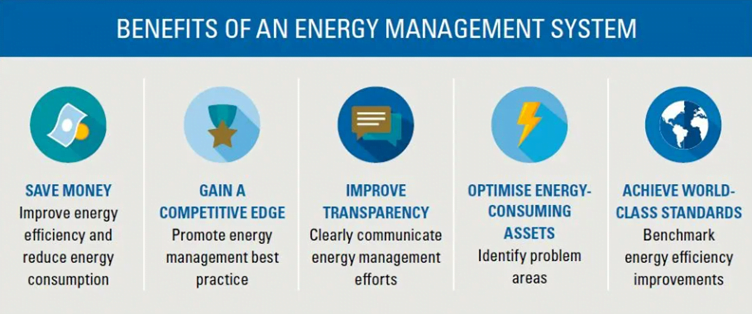
ISO 50001 – Energy Management System
ISO 50001 certification is not mandatory either but by becoming certified your company can have a strong energy management system that lowers energy use, has a less negative impact on the environment, and boosts profitability.
It outlines the requirements for management practices that are necessary to achieve better energy performance. This demonstrates that your company prioritises energy efficiency and that energy is controlled consistently.
By reducing energy use, you lower operating expenses and assist in developing a greener environment. This is the result of increased energy efficiency. For MedTech organisations to meet energy-related standards and show their dedication to sustainable practices, they must improve their environmental performance.
Strengthened compliance with energy-related regulations reduces the risk of non-compliance penalties and has the added benefit of giving a positive perception. Moreover, ISO 50001 certification increases market competitiveness, showcasing an organisation’s dedication to energy management and environmental responsibility.
Sustainability is a lifelong theme for manufacturers’ futures
Sustainability goes beyond how you manufacture your medical device. It includes many aspects such as how the business operates from people hiring, commuting etc to your offices, waste disposal and how facilities are powered (is it from renewable sources?). Also, travel behaviour, how the business operates and carbon footprint of it with distribution, company cars etc.
Clin-r+ recommendations
At this early stage, doing sustainability mapping of your medical devices is possible. Your clinical regulatory consultancy will need your bill of materials (BOM), manufacturing process and your economic operator map to do an interim LCA. This will highlight key areas for redesign for future iterations of your device to meet the sustainability requirements in MedTech’s future.
It’s recommended to start incorporating this thinking in your QMS processes and your Technical Documentation such as your design history file, risk management plan, PMS plan, clinical evaluation plan and clinical development plan.
Your consultancy will be able to help overcome this significant burden that is rapidly approaching the MedTech industry. But not only is you adaptation seamless and does not significantly work as usual but also gives you a key advantage in the marketplace as more and more procurement officers are tasked to compare similar technology and select eligible devices for tender based on sustainability scores making this a key area of market access that must be planned for by your sales and marketing depts.
Should you have any questions or need professional assistance, CLIN-r+ has a wealth of experience to call upon. Get in touch!