UKCA postponed for 1 year
What’s the opportunity for MedTech?
Medical device and IVD makers outside of the UK are relieved at the recent announcement that the UKCA deadline has been postponed. It indicates that there is additional time until the new market entry rules take effect to enter the UK market. This is outstanding news indeed for those manufacturers with an existing CE mark under MDD, MDR, IVDD, or IVDR, as they can use their current CE to ensure their access to the United Kingdom market.
Background to changes of the CE mark in UK
On December 31, 2020, at 11 p.m., the UK left the EU single market. The CE mark in Great Britain has been phased out since January 1st, 2021, and replaced by the United Kingdom Conformity Assessed (UKCA) mark. However, the CE mark will continue to be recognised for the majority of goods for a transitional period that ends on December 31, 2024.
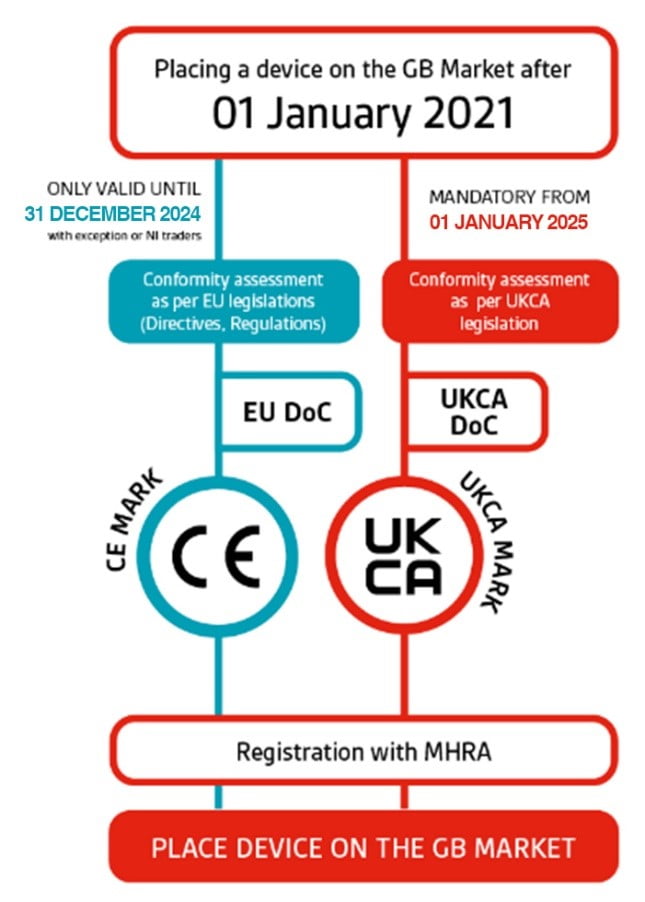
Which market does the UKCA cover - UK or Great Britain?
Manufacturers must make it clear EXACTLY where medical equipment are sold in the UK (Northern Ireland, England, Scotland, and Wales). The UKCA covers only Great Britain (GB), England, Scotland, and Wales.
Northern Ireland is exempt due to its adaptation of the EU CE criteria (EU MDR and IVDR deadlines). Therefore, further clarification is needed. Do the manufacturers sell in in Great Britain alone? In Northern Ireland? Or in both? Different rules apply, depending on their answer.
Figure 2: Different CE marks and the regions they apply to from 31 December 2024

Selling into Great Britain (England, Scotland & Wales)
To sell medical devices in Great Britain post-Brexit, manufacturers must;
- Have a current, valid CE mark in EU (MDD/MDR or IVDD/IVDR) OR compile a Technical File (TF) to outline how they meet the Essential Requirements for the EU MDD/IVDD.
- Meet the device registration deadline with the UK MHRA, which is a critical new obligation.
- Manufacturers outside the UK must identify and appoint a UK Responsible Person (UKRP), who must carry out this registration well before their devices’ registration deadline.
- Prepare to meet UKCA requirements by 31 December 2024.CE mark.
CLIN-r+ recommendations
We advise you obtain professional assistance to evaluate your application and technical file. With the help of your internal team, Clin-r+ can create a gap analysis of your current CE submission (such as 510K) and transfer overlapping data to create your UKCA technical file.
If you would like to know more about UKCA, we can provide professional assistance and clarify any issues. So, if you need help to compile a UKCA application or have any questions, CLIN-r+ has a wealth of experience to call upon. Get in touch!




