Medical Device Classification in Europe
The importance of getting it right
Going forward, medical device manufacturers must comply with the EU MDR before legally CE marking their products.
Before CE registration, your product’s medical device classification is crucial. Classification affects your device’s regulatory requirements, approval route, and expenditures.
Manufacturers are responsible for the correct classification of their device(s). This is an imperative step to ensure all other efforts in compiling your MDR submission is aligned. We’ve created this paper to give an overview of the process.
The illustration below shows the roadmap to obtain a CE mark.
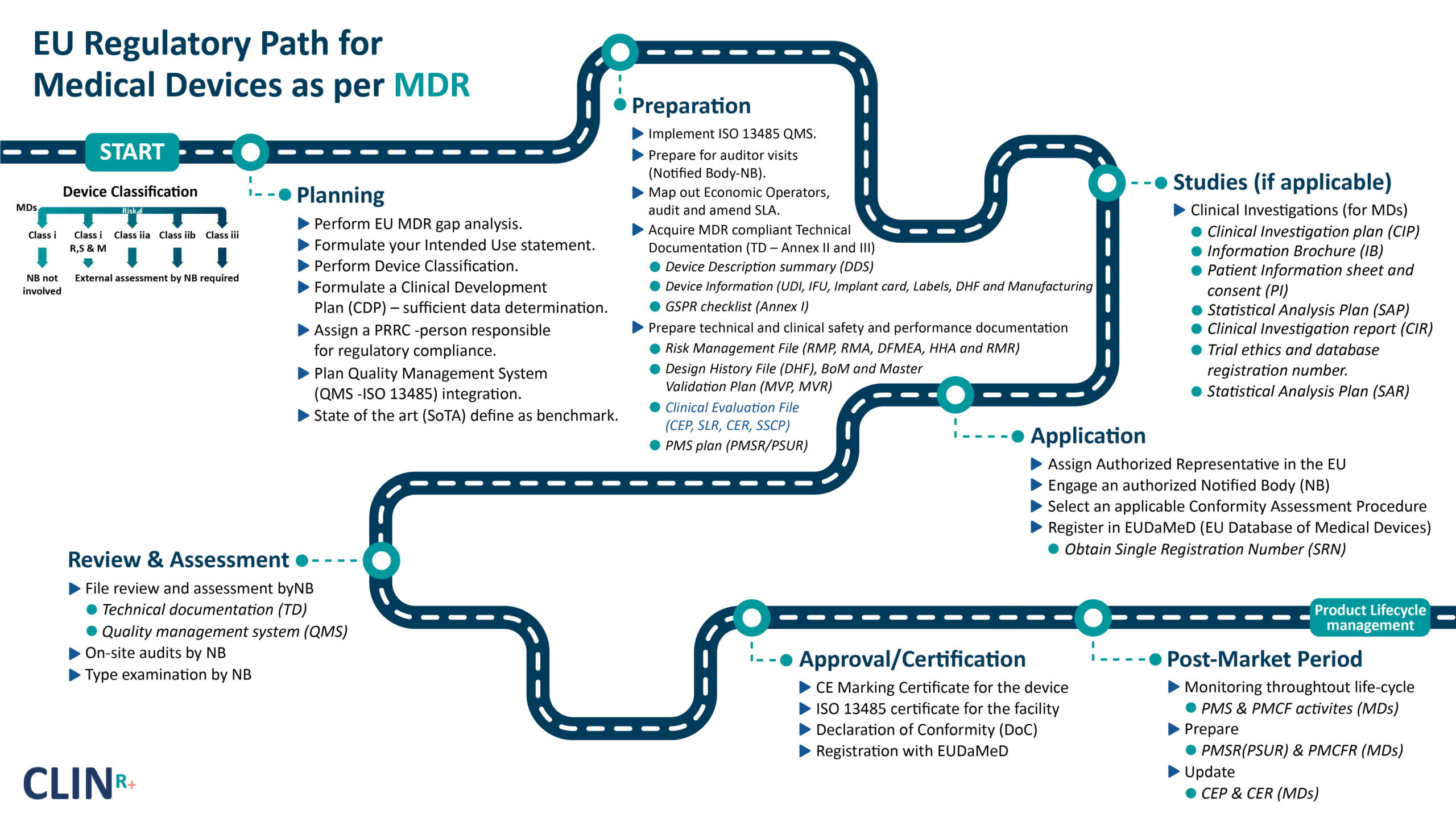
Determining your Device Classification for Europe
The European regulatory process begins with device classification under EU MDR 2017/745 for medical devices and active implantable medical devices or EU IVDR 2017/746 for IVD devices.
(Note: MDR also includes products specifically delineated that do not have a medical purpose – see Annex XVI).
Medical device classification in Europe is rule-based and the MDR Annex VIII has 22 rules. As the medical device risk increases, so does regulatory oversight and MDR compliance requirements.
(Note: The European approach differs from the U.S., which relies on FDA-approved predicates).
Medical Device Categories in Europe
Essentially, all devices fall into four basic categories:
- Non-invasive devices.
- Invasive medical devices.
- Active medical devices.
- Special Rules (including contraceptive, disinfectant, and radiological diagnostic medical devices).
However, the MDR also has some additional special rules, including one for nanomaterials.
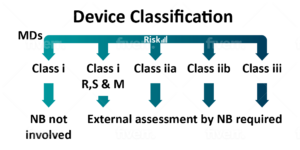
Medical Device Classification in Europe
Devices are segregated further into different classifications (listed below).
IVDs have their own classification system. IVD companies can also expect Notified Bodies to be more involved in the regulatory process than previously.
Under MDR, active implantable devices are still subject to similar requirements as Class III devices.
- Class I (low risk).
- Class Is (product that is delivered sterile).
- Class Im (product with a measuring function).
- Class Ir (products that are reprocessed).
- Class IIa (medium risk).
- Class IIb (medium/high risk).
- Class III (high risk).
CLIN-r+ recommendations
Consider partnering with clinical regulatory affairs consultancy who can help you determine the proper classification for your medical device. Whether you have a new device or you’re transitioning from MDD to MDR, or IVD to IVDR.
It’s important to note that once the correct risk has been identified, manufacturers still need to deal with residual safety and performance risks throughout the devices’ lifecycle. Risk Management and Post Market Surveillance are essential for this.
Manufacturers must also ensure that Technical Documentation demonstrates compliance with the MDR or IVDR.
Should you have any questions or need professional assistance, CLIN-r+ has a wealth of experience in medical device classification, risk management, post market surveillance and technical documentation to call upon. Get in touch!

