How to Apply Extractables and Leachables to Medical Devices
ISO 10993-1 changes have affected medical device biological safety testing. We no longer use the biocompatibility test matrix toxicity box because we now use materials, chemicals, analytical, and toxicological risk analyses.
ISO 10993-1:2018 is the name of the standard (which is not new). Medical device biological evaluation Part 1: Evaluation and testing within a risk management process should reflect the document’s philosophy. It instructs us to assess the risks and manage them. Every device now requires chemical knowledge, according to the new edition. It also instructed the driver to apply this knowledge to determine the device’s potential toxicity. The potential for toxicity becomes the driving force behind risk assessment, which may eventually lead to testing.
The idea is to list all your known materials, as well as any known toxicity data. You can then use this information to create a risk assessment for these materials, including any potential contaminants, as well as the device’s function. A manufacturer may decide no additional work is required if their product is non-invasive, has a short contact time, and is made of medically or food-grade materials. The need to chemically characterise the final product grows as the potential harm to the patient grows (due to increased invasiveness and/or longer contact). ISO 10993 part 18 and extractables and leachables become important at this point. You must use traditional in-vivo studies for toxicity end points if no other sources of information are available.
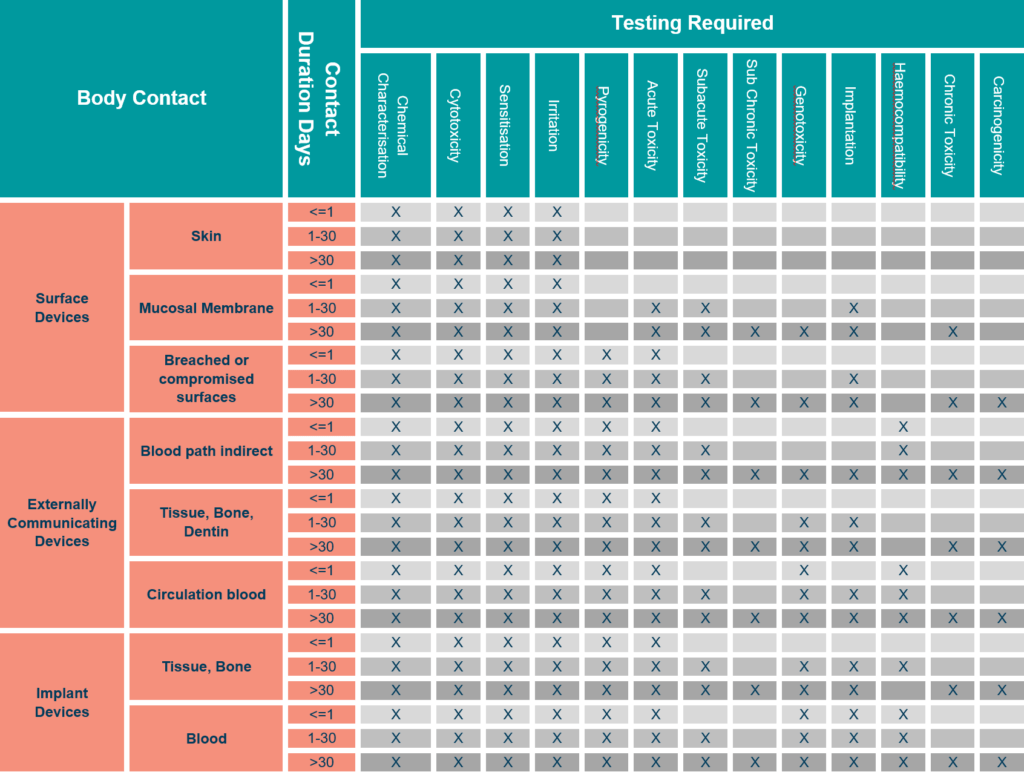
Biocompatibility Matrix
The ISO 10993-1 biocompatibility matrix now serves as a tool for determining which information requirements to use. Chemical analysis is now available in all categories. This does not always require testing, but also knowledge of potential toxicity.
When using the biocompatibility matrix philosophy, it is clear that we can tailor the rigour of chemical analysis and any associated toxicological risk analysis to the device’s application.
Therefore, continuous skin contact would require less intensive extraction and investigation of chemical migrants. A long-term implant, on the other hand, would require extensive extraction and analysis, as well as a thorough risk assessment of all materials identified.
Material Characterisation – Extractables and Leachables
The chemical materials present, as well as the morphology and nature of the surfaces, are all considered during material characterisation as described in ISO 10993-18. Surface features that encourage ingrowth or bacterial colonisation may be the focus of the surface investigation. Furthermore, there could be issues with the surface’s chemistry or catalytic properties. The investigation could use electron microscopy, elemental analysis, infrared spectroscopy, and other techniques.
The primary focus of the research will always be on the materials released by the medical device in use. This is the same concept used in traditional testing, which used device extracts in in-vivo testing. We know this as an extractables and leachables study in chemical analysis. The requirement for assessing materials migrating from a pharmaceutical container is fairly well defined. However, for medical devices, we must return to the standards and risk analyses.
ISO 10993-17
ISO 10993-17 defines leachables as “released constituents that may come into contact with an individual during clinical use.” The definition of ‘constituents that can be extracted in the laboratory’ includes additional entities that can be forced out of construction materials. In pharmaceutical container studies, the reason for identifying and quantifying the extractables is that they may transfer into the formulation during storage. Similarly, extractables in medical devices are being investigated because they may become leachable during the device’s lifetime.
When planning a study, remember that the manufacturing process may involve multiple chemicals (mould release, processing and machinery oils, solvents, UV or other adhesives). Many of the specified materials (especially plastics and adhesives) may also contain undeclared additives, such as activators/accelerators, catalysts, colours or enhancers, lubricants, scratch protection, side reaction products, residual monomers, UV or other stabilisers. The list is extensive.
 The concept of leachables works well in medical devices. Only dry skin is in contact with the adhesive on ECG electrodes. Only materials that could transfer to the skin over a few days are considered “in use leachables.” The patient is given a new dose of leachables if the electrode is replaced after three days. Forcing extractables from the electrode isn’t a good idea in this case. However, there is a risk of chemicals that are more difficult to extract, migrating into the patient with a long-term cerebral implant. “Simulated use” leachables is introduced in this section. Clearly, waiting many years for the extractable to migrate into solution for analysis is not an option.
The concept of leachables works well in medical devices. Only dry skin is in contact with the adhesive on ECG electrodes. Only materials that could transfer to the skin over a few days are considered “in use leachables.” The patient is given a new dose of leachables if the electrode is replaced after three days. Forcing extractables from the electrode isn’t a good idea in this case. However, there is a risk of chemicals that are more difficult to extract, migrating into the patient with a long-term cerebral implant. “Simulated use” leachables is introduced in this section. Clearly, waiting many years for the extractable to migrate into solution for analysis is not an option.
ISO 10993-12
ISO 10993-12 provides us with the extraction conditions (area to volume ratio, time and temperature, solvent polarity), but may not go far enough for more invasive devices.
As a result, forced extraction is used, with the strength of which (while based on ISO 10993-12) can be adjusted depending on the environment and usage duration. Hence, the term “simulated use extract.” We increase the solvent strength and consider increasing extraction times and temperatures as we progress up the invasiveness scale. Because we’re only interested in materials that will be used, not degradation products produced during the extraction process, keep this in mind.
Selection of Extractable and Leachable Analysis According to Patient Contact

Following the preceding analysis, it is logical to assign different levels of analysis to meet the requirements of the biocompatibility matrix. (We briefly described the analytical methods at the end of this document.)
1 – Medical Device Transient Contact
- Is suitable for surface contacting devices with transient or short-term use.
- Initial gathering of chemical data.
- Two extraction polarities, 37° C for 72 hours.
- Analysed according to standard protocols.
- Analyses GFAAF, GC-MS, HPLC-MS.
- Phthalate reference standard only.
- Toxicological risk analysis only if unexpected materials of concern are found.
2 – Medical Device Prolonged or Invasive Contact
- Is suitable for medium risk devices.
- Initial gathering of chemical data
- Material review.
- Two extraction polarities, 50° C for 72 hours.
- Analysed according to standard protocols with method verification.
- Phthalate reference standard, plus any substances referenced in material review.
- ICP-MS, GC-MS, GC/HS-MS, HPLC-MS Toxicological Risk Analysis.
3 – Medical Device Permanent Contact
- Implant and repeat use devices.
- Comprehensive gathering of chemical data MET.
- Material review and risk analysis.
- Specific study design and protocol using validated methods.
- Method development if required.
- Include comparative standards for materials of concern.
- Three extraction polarities, 50° C for 72 hours plus exhaustive or special extraction, sample concentration.
- Reference standards as specified in material review.
- May include degradation studies (ISO 10993-13).
- ICP-MS/AAF, GC/HS-MS, GC-MS, LC-MS, LC-TOF Toxicological Risk Analysis.
Table 1 – Standard surface areas and extract liquid volumes
Thickness mm | Extraction Ratio (surface area or mass/volume) ±10% | Examples of Forms of Materials |
<0,5 | 6 cm2/ml | Film, sheet, tubing wall |
0,5 to 1,5 | 3 cm2/ml | Tubing wall, slab, small-moulded items |
>1,0 | 3 cm2/ml | Larger moulded items |
>1,0 | 1,25 cm2/ml | Elastometric closures |
Irregularly shaped solid devices | 0,2 g/ml | Powder, pellets, foam, non-absorbent moulded items |
Irregularly shaped porous devices (low-density materials) | 0,1 g/ml | Membrane textiles |
Note: While there are no standardised methods available for testing absorbents and hydrocolloids, a suggested protocol is as follows:
| ||
Table 2 – Standard Extraction Conditions (ISO 10993-12)
Temperature | Extraction Time |
(37 ± 1) °C | 72 ± 2 hours |
(50 ± 2) °C | 72 ± 2 hours |
(70 ± 2) °C | 24 ± 2 hours |
(121 ± 2) °C | 1 ± 0.1 hours |
Toxicological Risk Analysis (TRA)
Analytical chemistry provides data on which materials are present and in what amounts. To be useful, this data must be interpreted in terms of the biocompatibility matrix’s toxicity end points. This can be a simple assessment if no materials of concern are found, or the patient contact is brief. As we identify more materials and patient contact becomes more intense, the need for a Toxicological Risk Analysis grows. A Registered Toxicologist is responsible for this analysis. Who calculates the patient dose per 24 hours and over the product lifetime for each material discovered? The potential toxicity of the materials, both individually and in combination, is then quantified using various data sources.
A Brief Description of Analytical Methods
LC-MS
Liquid chromatography – Mass spectroscopy is used to analyse liquid materials at normal temperatures. The toxicologist extracts these materials from a device or container using solvents. They then injected the solvent of the migrated materials into a tube containing a separation media, which separates the chemicals (this works like blotting paper and ink). Next, materials are individually presented to the mass spectrometer for mass analysis. The spectrometer uses technology similar to an old television with a cathode ray tube, and the electrons in the tube break down the molecules, which are then accelerated towards a target. How long it takes to arrive is proportional to the molecule’s mass and can be used to identify the substance.
MET also has a variant of this machine called an LC-TOF. The Time-of-Flight instrument is extremely accurate, allowing the identification of unknown materials by revealing their exact masses.
GC-MS
Gas chromatography – mass spectroscopy. This technology is based on the same principles as the LC, and at normal temperatures it examines gaseous or volatile materials.
GC-MS/HS
The instrument is a GC-MS with a heating system at the entrance point of the chromatography tube, which allows it to analyse materials with a lower boiling point (higher volatility) than those typically found in gas chromatography and liquid chromatography systems. It’s known as a Headspace GC-MS.
GFAAF
Graphite Furnace Atomic Absorption. An instrument that’s a lot of fun to play. It works in the same way that salt on a gas stove flame does, and the colour of the flame will change from blue to orange. The orange colour (or rather, its wavelength) is specific to the sodium in the salt. This method can be used to identify and quantify each metal’s unique colour range, and is how we learn about star composition, planetary atmospheres, and interstellar gases. The wavelength of light is stretched as stars move away from us, making it redder than we would expect for the “metals” present. So this “red shift” tells us how fast the stars are moving.
ICP-MS
Atomic absorption is similar to this analysis. This equipment is less sensitive, but it is more capable of detecting multiple metals.
CLIN-r+ top tips on being compliant:
ISO 10993 now requires knowledge of any chemicals released by a device while it is in use. We now include this in the testing matrix for each device category. Although materials characterisation isn’t the only way to get this information, it’s the most likely way to discover unexpected materials. We should tailor the rigour with which we apply chemical analysis to the device’s body contact and risk assessment. Through a Toxicological Risk Analysis (TRA), chemical information can often address most of the toxicity end points without animal testing.
Not all testing labs are equal, and your auditor will look at this. Ensure the labs you’re using have ISO 17025 accreditation and GLP. It’s also essential that they have a Toxicologist who will compile your Biocompatibility plan and undersign the TRA and BER.
We hope this document has answered your questions. Should you have any other questions or need professional assistance, CLIN-r+ can be a first point of contact to assess if your device needs biocompatibility testing and suitable labs. Get in touch!
References
- ISO 10993-1:2018: Biological evaluation of medical devices – Part 1: Evaluation and testing within a risk management process
- ISO 10993-18:2005: Biological evaluation of medical devices – Part 18: Chemical characterisation of materials
- ISO 10993-17:2002: Biological evaluation of medical devices – Part 17: Establishment of allowable limits for leachable substances
- ISO 10993-12:2012: Biological evaluation of medical devices – Part 12: Sample preparation and reference materials
- ISO 21726:2019: Biological evaluation of medical devices – Application of the threshold of toxicological concern (TTC) for assessing biocompatibility of medical device constituents
Remediating biocompatibility workflows to meet EU MDR compliance requirements
This case study looks at remediating biocompatibility workflows to meet EU MDR compliance requirements. CLIN-r+ supported the manufacturer with their problematic biocompatibility workflows to address …
Read More →

Biocompatibility
Recent changes to ISO 10993-1 have changed the landscape of medical device biological safety testing. The toxicity test box ticking from the biocompatibility test matrix …
Read More →

ISO 18562 – Biocompatibility evaluation
ISO 18562, has become the industry standard for testing the biocompatibility of breathing components. It has four parts: general principles, evaluation of particle emission, evaluation …
Read More →





