Claiming Equivalence under EU MDR
The comparison between MDD and MDR
By asserting equivalence on CE-marked devices, manufacturers can save time and money. However, tragedies involving devices such as breast implants and hip replacements have shown why manufacturers must be cautious when rushing to do equivalency submissions as skipping clinical studies means a safety reassurance has been removed in the registration process.
To guarantee the quality and safety of EU medical devices, the MDR went into effect in May 2021. The new legislation, which mandates CE marking for all products, has updated the certification process. Manufacturers can circumvent the time and expense of conducting clinical studies to satisfy the safety and performance requirements as outlined in the clinical evaluation by claiming equivalence with another device to get CE certification under the MDR. In teh path a manufacturer makes teh claim taht as teh equivalent device has clinical and their device is technically (same design), biologically (made from same material) and cllinically (used in the same clinical situation by same user) their device would have teh same expected patient outcomes.
Manufacturers of medical devices can utilise the technical documentation, post-market surveillance (PMS), post-market clinical follow-up (PMCF), and scientific literature associated with an already marketed device via the equivalency pathway.

Requirments for Demonstrating Equivalence
Equivalence under the MDR is still a contentious issue. It is important to assess if the equivalence strategy is applicable. Manufacturers must demonstrate:
- “sufficient levels of access to the data”
- “sufficient clinical data in Clinical Evaluation”
- Device eligable for equivalence route
Demonstration of “sufficient levels of access to the data” required to justify claims of equivalence
In addition to outlining the technical, biological and clinical characteristics to be taken into consideration for the demonstration of equivalence, Annex XIV Section 3 MDR requires manufacturers to have “[…] sufficient levels of access to the data relating to devices with which they are claiming equivalence in order to justify their claims of equivalence”. As clarified above, demonstration of “sufficient levels of access” does not require a contract in all circumstances. A contract is only required for the exemption case described in Article 61(5). It should also be noted that Annex XIV Section 3 refers specifically to the data required to justify claims of equivalence: i.e., the requirement is for sufficient access to establish the clinical, technical and biological characteristics against which equivalence is evaluated, not access to the complete technical documentation.
The MDR demands more product equivalence documentation than the Medical Device Directive (MDD). The Medical Device Regulations will require technical, biological, and clinical parameters for claiming equivalence.
MDR Annex XIV Part A (3) states that if you can prove equivalence, a clinical review may use clinical data of a product that somebody has already commercialised. Every feature that the manufacturer claims to be equal must be supported by appropriate facts and evidence. We use technical, biological, and clinical aspects to show equivalence. Each must exhibit the following similarities.
- Technical: similarities in design, usage, specifications, properties, deployment methods, principles of operation and critical performance requirements.
- Biological: similarities in the materials/substances, human tissues/fluids, duration, and way of contact with the subject.
- Clinical: similarities in clinical condition, severity and stage of disease, user, clinical effect and patient’s characteristics (e.g. weight, age, gender, physiology).
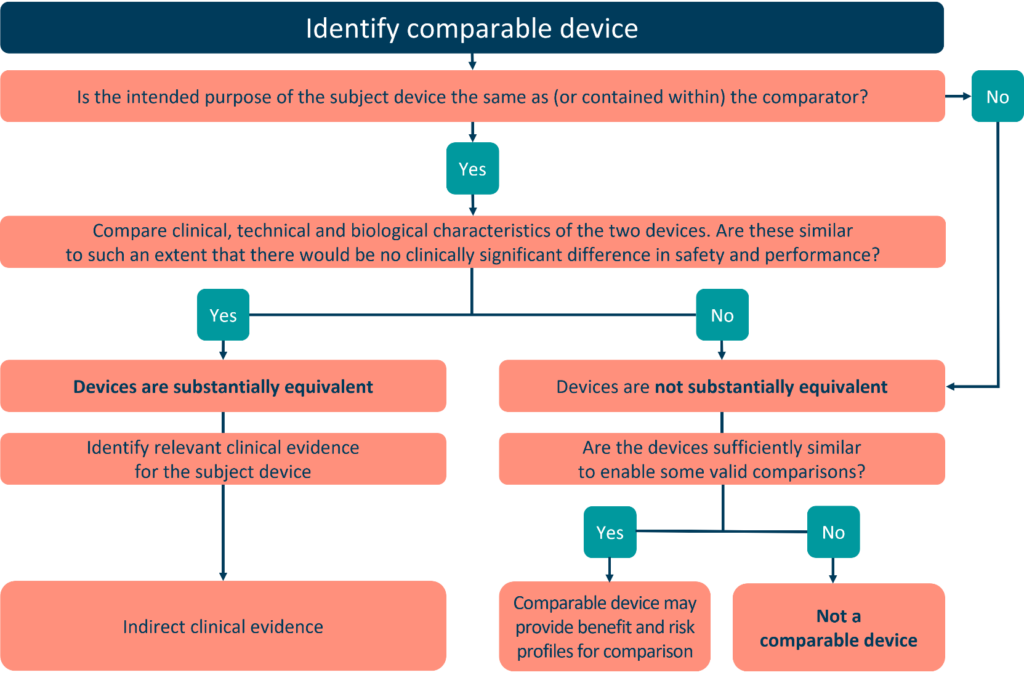
When proving equivalency, we must evaluate all three traits in their entirety. They must be comparable to the point where there is no clinically discernible difference between the two devices’ clinical performance and safety. Scientific evidence must support equivalence considerations.
We cannot compare two devices with identical features, but built of different materials. As a result, a comprehensive literature review, gap analysis, and additional clinical investigations are required.
Equivalence does not exempt a device from having to perform a clinical evaluation, which is a requirement of MDR. If a manufacturer cannot establish appropriate levels of access to presumed equivalent device data then they cannot claim equivalence for clinical evaluation. Additionally, it is not acceptable to assert equivalence by using different parts of devices (the Frankenstein approach).
We must study, document, and demonstrate each claimed equivalent device in the Clinical Evaluation Report (CER).
Class III and implantable devices requirements
Manufacturers of class III and implantable medical devices may avoid clinical examination under MDR Article 61(4) if they can establish the following equivalence:
- modifications of a device already marketed by the same manufacturer have designed the device,
- equivalence can be demonstrated according to the MDR between the devices, and
- the clinical evaluation of the marketed device is sufficient to demonstrate conformance with the applicable safety and performance requirements.
Article 61(4) of Regulation (EU) 2017/745 on medical devices (MDR) requires clinical investigations to be performed for implantable and class III devices, except in four specific cases as outlined in: CASE 1) indents 1-3 of Article 61(4); CASE 2) Article 61(6)(a); CASE 3) Article 61(6)(b); CASE 4) Article 61(5) in MDCG 2023-7.
According to MDR Article 61(5), the manufacturer of implantable and class III devices that claim to be equivalent to a product already commercialised, but not made by the same company, must have a contract to grant continuing access to the product’s technical and clinical documentation. In this case, the equivalent device must have passed its initial clinical examination in accordance with MDR and be MDR certified. According to the MDCG 2020-5, MDD-certified devices cannot be used to assert equivalence.
According to the MDCG 2023-7, provides a summary of the cases when implantable and class III devices may be exempted from mandatory clinical investigations.
CASE 1:
Indents 1-3 of Article 61(4)
• DUE has been designed by modifications of a device already marketed by the same manufacturer.
• Equivalence is demonstrated between the DUE and the manufacturer’s ED in accordance with Section 3 of Annex XIV; demonstration of equivalence has been endorsed by the notified body. For further guidance on the demonstration of equivalence,
please refer to MDCG 2020-5.
• The clinical evaluation of the marketed device is sufficient to demonstrate conformity of the modified device with the relevantsafety and performance requirements4, 5
• PMCF plan is appropriate and includes post market studies to demonstrate the safety and performance of the DUE6
CASE 2:
Article 61(6)(a)
• DUE has been lawfully placed on the market or put into service in accordance with Directive 90/385/EEC or Directive 93/42/EEC.
• The clinical evaluation is based on sufficient clinical data
• The clinical evaluation is in compliance with the relevant productspecific CS for the clinical evaluation of that kind of device, where such a CS is available.
CASE 3:
Article 61(6)(b)
- DUE is one of the listed types of devices: “sutures, staples, dental fillings, dental braces, tooth crowns, screws, wedges, plates, wires, pins, clips or connectors”.
- The clinical evaluation is based on sufficient clinical data
- The clinical evaluation is in compliance with the relevant product specific CS for the clinical evaluation of that kind of device where such a CS is available.
CASE 4:
Article 61(5)8
- Equivalence is demonstrated between the DUE and the other manufacturer’s ED in accordance with Section 3 of Annex XIV; demonstration of equivalence has been endorsed by the notified body (via Article 61(4)).
- The clinical data from the clinical evaluation of the ED is sufficient to support the intended purposes of the DUE (via Article 61(4))9
- The two manufacturers have a contract in place that explicitly allows the manufacturer of the ED full access to the technical documentation on an ongoing basis.
- The clinical evaluation of the other manufacturer’s ED has been performed in compliance with the requirements of the MDR10, 11
- PMCF plan is appropriate and includes post market studies to demonstrate the safety and performance of the DUE6 (via Article 61(4)).
Other device class requirements
It is unspecified if the equivalent device for class III and non-implantable devices must be marketed in the EU (Article 61(3)). MDCG 2020-5 permits the claim of equivalence for MDD, MDR, and even non-CE designated devices. But the device must fulfil all relevant MDR requirements regarding equivalence and clinical evaluation.
Non-CE-marked device requirements include:
- The manufacturer must have sufficient levels of access to the data.
- Manufacturers conducted clinical investigations following good clinical practices (ISO 14155).
- Clinical data meets the MDR requirements and is transferrable to the EU.
A contract between the manufacturers to control access to the technical documentation is not necessary if another company makes the equivalent equipment.
Clinical evaluation can use clinical data of a non-CE-marked device, according to MEDDEV 2.7/1 Rev 4. This is similar to the advice in MDCG 2020-5 and MDCG 2023-7.
MEDDEV 2.7/1 Rev 4 vs EU MDR
MDCG 2020-5 clarifies equivalency and contrasts MDR and MEDDEVV 2.7/1 Rev.4. In terms of technical, biological, and clinical equivalency, it also establishes requirements for clinical evidence.
Table 1: MDR vs MEDDEVV 2.7/1 Rev 4
| Equivalence | MDR, Annex XIV Part A (3) | MEDDEV 2.7/1 rev 4, Appendix A1 |
Technical | The device is of similar design; is used under similar conditions of use; has similar specifications and properties including physicochemical properties such as intensity of energy, tensile strength, viscosity, surface characteristics, wavelength and software algorithms; uses similar deployment methods, where relevant; has similar principles of operation and critical performance requirements. | Be of similar design, and – Used under the same conditions of use, and – have similar specifications and properties (e.g. physicochemical properties such as type and intensity of energy, tensile strength, viscosity, surface characteristics, wavelength, surface texture, porosity, particle size, nanotechnology, specific mass, atomic inclusions such as nitrocarburizing, oxidability), and – use similar deployment methods (if relevant), and – have similar principles of operation and critical performance requirements. |
Biological | The device uses the same materials or substances in contact with the same human tissues or body fluids for a similar kind and duration of contact and similar release characteristics of substances, including degradation products and leachables. Exceptions can be foreseen for devices in contact with intact skin and minor components of devices. In these cases, risk analysis results may allow the use of similar materials, taking into account the role and nature of the similar material. | Use the same materials or substances in contact with the same human tissues or body fluids. Exceptions can be for devices in contact with intact skin and minor components of devices. In these cases, risk analysis results may allow the use of similar materials, taking into account the role and nature of the similar material. |
Clinical | The device is used for the same clinical condition or purpose, including similar severity and stage of disease, at the same site in the body, in a similar population, including age, anatomy and physiology; has the same kind of user; has similar relevant critical performance in view of the expected clinical effect for a specific intended purpose. | – used for the same clinical condition (including when applicable similar severity and stage of disease, same medical indication), and – used for the same intended purpose, and – used at the same site in the body, and – used in a similar population (this may relate to age, gender, anatomy, physiology, possibly other aspects), and – not foreseen to deliver significantly different performances (in the relevant critical performances, such as the expected clinical effect, the specific intended purpose, the duration of use, etc.) |
Conclusion | The characteristics listed in the first paragraph shall be similar to the extent that there would be no clinically significant difference in the safety and clinical performance of the device. Considerations of equivalence shall be based on proper scientific justification. | For assuming equivalence, – all three characteristics (clinical, technical, biological) need to be fulfilled. Similar means that no clinically significant difference in the performance and safety of the device would be triggered by the differences between the device under evaluation and the device presumed to be equivalent. |
MDCG 2020-5 Annex I offers an equivalence table. All technical, biological, and clinical characteristics are listed in the table for assessment. The additional column is to include the findings of the assessment and any differences found. In conclusion, the table should provide scientific support for the claim that there won’t be clinically significant variations in the safety and clinical effectiveness of the device.
Equivalent vs Similar
MDCG 2020-5 also distinguishes between equivalent and similar.
We may deem the device equivalent if we prove that the clinical, technological, and/or biological variations have no clinically discernible impact on the performance and safety of the device. To show equivalence, the discrepancies between the two devices must be negligibly different. The claim needs to be supported by pre-clinical (bench testing or in vivo investigations) and/or clinical (clinical investigation or post-market) data.
Numerous or significant variances will compromise equivalence claims. The devices are seen as being similar in this instance.
The manufacturer must ensure that all pertinent information about the comparable product is available for clinical evaluation. It must prove its effectiveness and safety.
CLIN-r+ Recommendations
In order to demonstrate equivalence thoroughly:
- Use tables to compare and contrast the two devices to prove equivalence.
- Clearly state all device differences.
- Critically analyse these differences to determine their likely safety and performance implications.
- Illustrate device similarities and differences.
Even with the additional requirements for class III devices, medical device manufacturers can still prove equivalence. They can use clinical data from equivalent devices to optimise their CE-marking process.
New device manufacturers must analyse potentially equivalent devices soon before the transition phase ends.
Gap assessments of medical devices that were CE marked under MDD are required. This should take previous equivalency analyses and related clinical data into consideration.
Clin-r+ Ltd can assist medical device manufacturers with gap assessments, the transition from MDD to MDR, and the demonstration of equivalence to comply with MDR requirements. Get in touch!






