Clinical Evaluation Conformity Routes
EU MDR, MDCG 2020-6, MDCG 2023-7 and MDCG 2024 compliance
Conformity routes under the clinical data needs for the EU MDR are not well understood and can lead to unnecessary costs due to investment into clinical investigations or unsuccessful CE assessments. Adequate advice and planning for clinical evaluations can save time, money and unnecessary worry and should be an activity undertaken at the initiation of a new device or when the transition to MDR is planned.
Before placing a medical device on the EU market, the manufacturer must demonstrate to their notified body that the device is safe for use and performs as intended. In other words, the medical device does not cause unexpected harm to a patient during normal use and functions as expected. A clinical evaluation report (CER) is originated by the manufacturer documents with their devices’ supporting clinical evidence and conclusions regarding the safety and performance of the device. This is a key document submitted to the notified body in support of CE marking (Medical Device Coordination Group, 2021a). Under European Medical Device Regulations clinical evaluation is defined as “a systematic and planned process to continuously generate, collect, analyse and assess the clinical data pertaining to a device in order to verify the safety and performance, including clinical benefits of the device when used as intended by the manufacturer” (EUR-Lex, 2021).
In this paper, we outline the clinical evaluation route options, their requirements, and which devices they apply to.
Article 61(3) – Clinical Investigations to acquire safety and performance clinical data
This route of clinical evaluation conformity is by far the most expensive, risky and time consuming. It is necessary when there is a new novel device that the following requirements are met.
Requirements:
(a) Must have Clinical Investigation plan that meets ISO 14155
(b) Must meet GCP
(c) May need ethical and Competent Authority clearance before investigations start, dependent on country regulations for clinical trials.
Applicable devices:
- Pre-market devices without a legacy device
- Medical devices without clinical data
- Novel MD that does not meet definition for well established technology (WET)
- Brand new devices under MDR, that cannot claim equivalence due to lack of access to TD of a suitable equivalent device.
- Legacy devices without sufficient clinical evidence and that do not meet WET
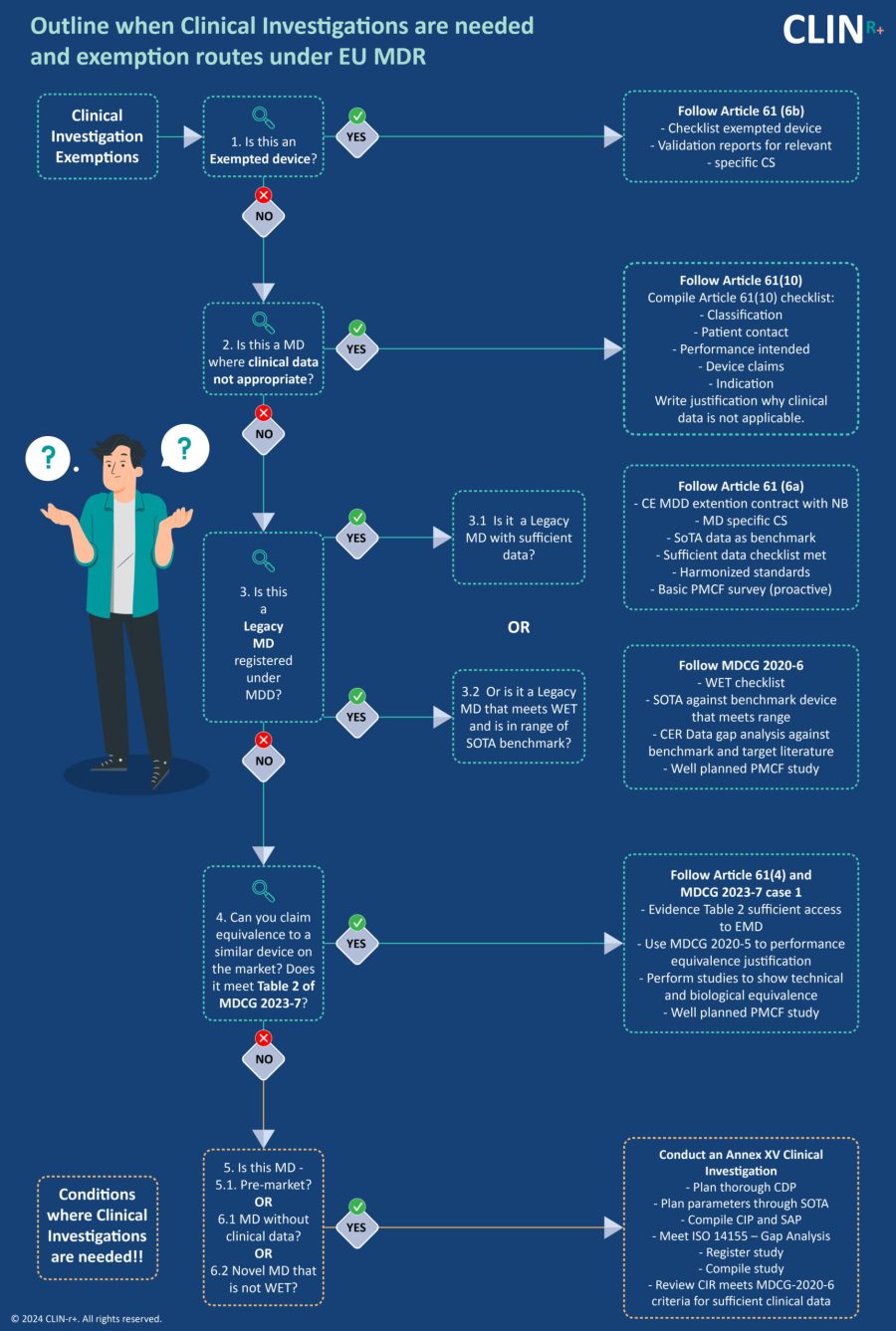
Article 61(4) Modified device that is equivalent to own device
Exception for Implantable and class III devices who don’t want to perform a clinical investigation.
Requirements:
- The manufacturer has made modifications to a device which they have already marketed (either under EU MDD or MDR).
- Manufacturers can claim equivalence to a device they already market.
- Notified Body agrees with the equivalence claim.
- Clinical evaluation of the marketed device is sufficient to demonstrate conformity to the GSPRs (CER is MDR compliant).
- Manufacturers must perform a PMCF study and demonstrate the safety and performance of the device to be CE marked.
Applicable devices:
- Implantable devices
- Class III devices
Legacy devices without sufficient clinical evidence and that do not meet WET but meet EU MDR requirements.
Article 61(5) Equivalence to competitor device that is MDR compliant
Exception for Implantable and class III devices who do not want to perform a clinical investigation and have access to TD to a suitable equivalent device.
Requirements:
- Manufacturers can claim equivalence to another manufacturer’s device, but it must already be CE marked under the MDR.
- A contract must be put in place that explicitly allows the manufacturer full access to the technical documentation of the equivalent device on an ongoing basis.
- The clinical evaluation must be performed in accordance with MDR requirements, and the CER of the equivalent device must be MDR compliant.
- Manufacturer needs to provide clear evidence to the Notified Body.
Applicable devices:
- Implantable devices
- Class III devices
- Legacy devices without sufficient clinical evidence and that do not meet WET.
- New devices that have a suitable equivalent in the market that meets MDR requirements.
Article 61(6a) - Legacy MDD device with sufficient clinical data
Devices that have a MDD certificate and during the MDD period acquired sufficient clinical data to prove the safety and performance of the device as outlined in MDCG 2020-6.
Requirements:
- Clinical evaluation must be based on sufficient clinical data (as per MDCG 2020-6).
- Compliance with the relevant product specific common specification (CS) must be shown. In the absence of CS, manufacturers must prove sufficient clinical evidence.
Applicable devices:
- Legacy implantable devices
- Class III devices
Article 61(6b) Exempted devices
If your device is a suture, staple, dental filling, dental braces, tooth crowns, screws, wedges, plates, wires, pins, clips, connectors.
Requirement:
- Manufacturers must base their clinical evaluation on sufficient clinical data (as per MDCG 2020-6).
- Manufacturers must be compliant with the relevant common specification (CS).
- Compliance with the relevant product specific common specification (CS) must be shown. In the absence of CS, manufacturers must prove sufficient clinical evidence.
Applicable devices:
- WET devices
TO NOTE
It should be noted that a manufacturer can claim article 61 (6a & 6b) with no common specification (CS) in existence at the time of CE marking. However, if the relevant CS becomes available or released post CE marking, then the manufacturer must update their technical documentation to comply with the relevant common specifications. If this isn’t done then they could lose the CE mark.
Article 61(9) MD with no medical purpose (Annex XVI devices)
For devices with no medical purpose (Annex XVI devices)
Requirement:
- Manufacturers must demonstrate a clinical benefit in accordance with this Chapter and Annexes XIV and XV shall be understood as a requirement to demonstrate the performance of the device.
- Clinical evaluations of those products shall be based on relevant data concerning safety, including data from post-market surveillance, PMCF, and, where applicable, specific clinical investigation.
- Clinical investigations shall be performed for those products unless reliance on existing clinical data from an analogous medical device is duly justified.
NOTE: As per MDR Article 61(9), manufacturers may either perform a clinical investigation for these Annex XVI devices or claim reliance on an analogous medical device.
Applicable devices:
- Annex XVI devices (listed below)
- Contact lenses or other items intended to be introduced into or onto the eye.
- Products intended to be totally or partially introduced into the human body through surgically invasive means for the purpose of modifying the anatomy or fixation of body parts with the exception of tattooing products and piercings.
- Substances, combinations of substances, or items intended to be used for facial or other dermal or mucous membrane filling by subcutaneous, submucous or intradermal injection or other introduction, excluding those for tattooing.
- Equipment intended to be used to reduce, remove or destroy adipose tissue, such as equipment for liposuction, lipolysis or lipoplasty.
- High intensity electromagnetic radiation (e.g. infra-red, visible light and ultra-violet) emitting equipment intended for use on the human body, including coherent and non-coherent sources, monochromatic and broad spectrum, such as lasers and intense pulsed light equipment, for skin resurfacing, tattoo or hair removal or other skin treatment.
- Equipment intended for brain stimulation that apply electrical currents or magnetic or electromagnetic fields that penetrate the cranium to modify neuronal activity in the brain.

Article 61(10) MD where clinical data is not appropriate
When demonstration of conformity with general safety and performance requirements (GSPRs) based on clinical data isn’t deemed appropriate. Manufacturers must provide the following.
Requirements:
- Adequate justification based on manufacturer’s risk management results, device-human interaction, intended clinical performance, and manufacturer claims.
- The manufacturer must justify the device technical documentation (referred to in Annex II) why non-clinical testing methods, such as performance evaluation, bench testing, and pre-clinical evaluation, alone are sufficient for demonstrating conformity with general safety and performance requirements.
- Only low-risk devices with no clinical benefit will be considered, as they do not positively impact individual health in terms of meaningful, measurable clinical outcomes, diagnosis, patient management, or public health. Lab fridges, scales for blood products, and others may be considered under this topic.
Applicable devices:
- Low risk devices with no clinical benefit
NOTE: Not applicable to Class III and implantable devices.
Article 61(6a) - Legacy MDD device with sufficient clinical data (MDCG 2020-6 Section 1.2)
Legacy devices claiming Well Established Technology (WET) must meet the requirements below and provide a detailed rationale and supporting documents for each criteria. WET devices all have the following.
Requirements:
- Relatively simple, common, and stable designs with little evolution.
- Their generic device group has well-known safety and has not been associated with safety issues in the past.
- Well-known clinical performance characteristics and their generic device group are standard of care devices where there is little evolution in indications and the state of the art.
- A long history on the market.
Applicable devices:
- Legacy devices claiming Well Established Technology (WET).
MDCG 2020-6 Appendix III
According to MDCG 2020-6, appendix III table, a manufacturer must specify the amount of evidence for a WET device. This must show what level of evidence has been provided based on the table below.
Complaints and vigilance alone are insufficient.
Rank | Types of clinical data and evidence | Considerations / comments |
1 | Results of high quality clinical investigations covering all device variants, indications, patient populations, duration of treatment effect, etc. | This may not be feasible or necessary for certain well-established devices with broad indications (eg Class IIb legacy sutures, which could be used in every conceivable patient population). |
2 | Results of high quality clinical investigations with some gaps. | Gaps must be justified / addressed with other evidence in line with an appropriate risk assessment, and clinical safety, performance, benefit and device claims. Assuming gaps can be justified, there should be an appropriate PMCF plan to address residual risks. Otherwise, manufacturers shall narrow the intended purpose of the device until sufficient clinical data has also been generated. |
3 | Outcomes from high quality clinical data collection systems such as registries. | Is there sufficient evidence of the quality of the data collected by the registry? Are the devices adequately represented? Is the data appropriately stratified? Are the endpoints appropriate to the safety, performance and endpoints identified in the clinical evaluation plan? |
4 | Outcomes from studies with potential methodological flaws but where data can still be quantified, and acceptability justified. | Many literature sources fall into this category, due to limitations such as missing information, publication bias, time lag bias, etc. This applies equally to publications in the peer-reviewed scientific literature. However, for legacy devices where no safety or performance concerns have been identified, these sources can be sufficient for confirmation of conformity to the relevant GSPRs is appropriately appraised and the gaps are identified and handled. High quality surveys may also fall into this category. |
Note: Class III legacy devices and implantable legacy devices which are not well-established technologies should have sufficient clinical data as a minimum at level 4. Those devices which are well-established technologies may be able to confirm conformity with the relevant GSPRs via an evaluation of cumulative evidence from additional sources as listed below. Reliance solely on complaints and vigilance is not sufficient. | ||
5 | Equivalence data (reliable / quantifiable). | Equivalence must meet MDR criteria. It is normally expected that manufacturers should gather data on their own devices in the post-market phase, therefore reliance on equivalence should be July justified, and linked to appropriate PMCF or proactive PMS. |
6 | Evaluation of state of the art, including evaluation of clinical data from similar devices as defined in SoTA. | This is not considered clinical data under the MDR, but for well-established technologies only can be considered supportive of confirmation of conformity to the relevant GSPRs. Data from similar devices may also be important to establish whether the device under evaluation and similar devices belong to the group of devices considered as WET. See MDCG 2020-6 Section 1.2 for the criteria for WET. Data from similar devices may be used, for example, to demonstrate ubiquity of design, lack of novelty, known safety and performance profile of a generic group of devices, etc. |
7 | Complaints and vigilance data; curated data. | This falls within the definition of clinical data under MDR Article 2(48), but it is not generally considered a high quality source of data due to limitations in reporting. It may be useful for identifying safety trends or performance issues. High volume data collected within a robust quality system may provide supportive evidence of device safety. |
8 | Proactive PMS data, such as that derived from surveys. | This falls within the definition of clinical data under MDR Article 2(48), but it is not generally considered a high quality source of data due to limitations associated with sources of bias and quality of data collection. It may be useful for identifying safety concerns or performance issues. |
9 | Individual case reports on the subject device. | This falls within the definition of clinical data under MDR Article 2(48), but it is not generally considered a high quality source of data due to limitations in generalizing findings to a wider patient populations, reporting bias, etc. It may provide supportive or illustrative information with respect to specific claims. |
10 | Compliance with non-clinical elements of common specifications considered relevant to device safety and performance. Common specifications may address clinically relevant endpoints through non-clinical evidence such as mechanical testing for strength and endurance, biological safety, usability, etc. | Common specifications which address clinical investigations or data requirements directly would rank higher in this hierarchy. Common specifications may address clinically relevant endpoints through non-clinical evidence such as mechanical testing for strength and endurance, biological safety, usability, etc. |
11 | Simulated use / animal / cadaveric testing involving healthcare professionals or other end users, | This is not clinical data, but maybe considered evidence of confirmation of conformity to relevant GSPRs, particularly in terms of usability, such as for accessories or instruments. |
12 | Pre-clinical and bench testing / compliance to standards. | Pre-clinical and bench testing may address clinically relevant endpoints through non-clinical evidence such as mechanical testing for strength and endurance, biological safety, usability, etc. |
Important Information before submission
Information for all legacy devices
Legacy devices on the market that have been subject to conformity assessment are presumed to have clinical data, under MDCG 2020-6. Post-market clinical data and clinical data from the MDD/AIMDD conformity assessment will be used to evaluate legacy devices under the MDR. Manufacturers must state what clinical evaluation route (equivalence and/or clinical investigation) was used during the initial conformity assessment.
For legacy devices, manufacturers must specify the clinical evaluation route that was used during the devices’ initial conformity assessment when the device was first CE marked. E.g equivalence, clinical investigation, or both equivalence and clinical investigation.
Information for all legacy devices which previously claimed equivalence during their initial assessment (when the device was first CE marked)
If the clinical evaluation route during the initial conformity assessment (when the device was first CE marked) was based on equivalence, and you have not presented an equivalent device/argument to meet the MDR requirements, or no clinical investigation(s) have been performed during this MDR submission, the below statements shall apply during the review of your file.
According to MDCG 2020-6 Section 5, page 9 of 22, the European Commission guidance MEDDEV 2.12/2 on PMCF states that the certifying notified body must verify PMCF studies if clinical evaluation was based exclusively on clinical data from equivalent devices for initial conformity assessment.
Information for all devices with new MDR application (not legacy) claiming equivalence
All MDR new applications (not legacy devices) claiming equivalence must have a PMCF study plan as per MEDDEV 2.12/2. Manufacturers must include a PMCF clinical study plan in the device submission.
CLIN-r+ recommendations
When clinical data is insufficient to cover all devices, intended use, safety, and performance claims, manufacturers should conduct a gap analysis based on MDR standards. Data gaps can be filled in several ways. PMCF studies may be the best way to gather clinical evidence, although scientifically sound questionnaires or registries can also be used. Manufacturers might limit the device’s intended use until clinical data is available.
Manufacturers without this expertise should consider partnering with an experienced consultancy when assessing standards, risk, and clinical data and considering what clinical studies are best suited for your devices’ CE marking needs. Consultancies also come with resources such as clinical study designers, statisticians and experienced SoTA medical writer’s systematic review helping expand your companies’ capabilities cost-effectively.
Clin-r+ provides expert assistance and has a wealth of experience to call upon. We can assist your transition and ensure you are MDR compliant. To learn more about our services and how we can help. Get in touch

