Intended Purpose, Intended Use, and Indications for Use
The difference between these terms in a nutshell
Manufacturers often confuse Intended Purpose, Intended Use and Indications for Use. Not only do they sound similar, but their definitions in relation to a device can be easy to mix up.
Indications for Use and Intended Purpose are actually the same. The US market more commonly uses the term ‘indications for use’. However, the EU MDR uses the term ‘intended purpose’.
Intended Purpose and Intended Use are both terms that medical device manufacturers should take time to understand. They affect various aspects of device design, development, and regulatory pathways.
Definitions
The definitions are as follows:
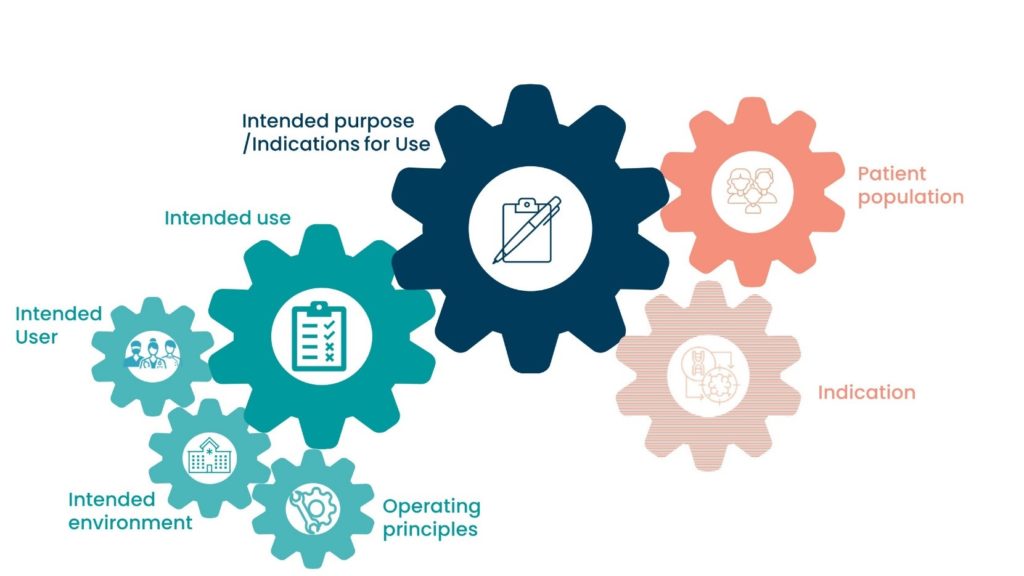
Intended Purpose / Indications for Use
The use for which the device is intended according to the data supplied by the manufacturer on the labelling, in the instructions and/or in promotional material.
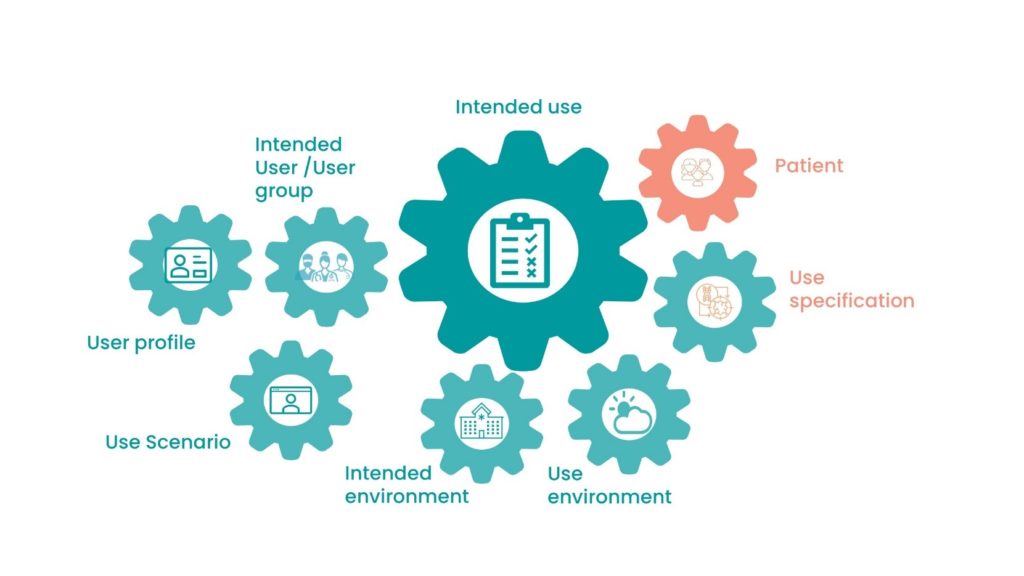
Intended Use
Focuses on the medical purpose.
The medical indication of the product, process, or service.
The medical procedure we use the device in.

So, what does that actually mean?
Intended Purpose / Indications for Use should state key information regarding the device. This should include:
- The procedure it is intended to be used for.
- What the device is used to do.
- The devices mode of action.
- The device indication(s).
- Who the patient population is.
- Who the intended user of the device is.
Your intended purpose must also have relevant documentation to back it up. This could be in the form of certificates or clinical data, for example.
Intended Use provides more detail on how the device is used with different focus. This should include:
- What type of device it is.
- What field of medicine it’s used in.
- If the device is sterile/non-sterile.
- Whether the device is reusable, single use, etc.
The risk classification of a device is a function of its intended use. Manufacturers should ensure that both statements for Intended Purpose and Intended Use are clear and definitive.
Not all devices need to define of all of the terms stated in the EU MDR. There are exceptions, as not all devices have a specified condition or population that it treats (indications). However, all devices have an Intended Use and Intended Purpose. For example, we use a scalpel in so many conditions or patient groups with a limited use (e.g., only creating the incision during the procedure) and in various procedures (e.g., colorectal surgery, laparoscopic investigations). In this case, it wouldn’t be practical or possible to have an ”Indication” and add an indication statement within the intended purpose.
Indications
It is important to note that not all devices have indications, as clarified by the definition of ‘indication’ in MDCG 2020-6:
‘indication’, ‘indication for use’: refers to the clinical condition that is to be diagnosed, prevented, monitored, treated, alleviated, compensated for, replaced, modified, or controlled by the medical device. We should distinguish it from ‘intended purpose/intended use’, which describes the effect of a device. All devices have an intended purpose/intended use, but not all devices have an indication (e.g. medical devices with an intended purpose of disinfection or sterilisation of devices).
Indications for use are about the patient and should answer questions like:
- What illness, injury, disease, or condition is the device intended to prevent, diagnose, or treat?
- Under what circumstances would someone use the device?
- What is the target population? Where (anatomically) will it be used? What duration will they use it for?
Manufacturers should also note that your indications for use can also change the regulatory pathway for your device.
For instance, we consider a scalpel a ‘Surgical Invasive device’ because as per the regulations, it is (a) an invasive device which penetrates inside the body through the surface of the body, including through mucous membranes of body orifices with the aid or in the context of a surgical operation; and (b) a device which produces penetration other than through a body orifice. So initially it is a Class IIb.
However, if we broaden the device’s indication or intended use to the central nervous system or circulatory system, then Classification Rule 7 applies, and it can upgrade your device from a Class IIb to Class III.
“Rule 7 – All surgically invasive devices intended for short-term use are classified as class IIa unless they are:
— intended specifically to control, diagnose, monitor or correct a defect of the heart or of the central circulatory system through direct contact with those parts of the body, in which case they are classified as class III;
— specifically intended for use in direct contact with the heart or central circulatory system or the central nervous system, in which case they are classified as class III;
— intended to supply energy in the form of ionizing radiation in which case they are classified as class IIb;
— have a biological effect or are wholly or mainly absorbed in which case they are classified as class III;
— are intended to undergo chemical change in the body in which case they are classified as class IIb, except if the devices are placed in the teeth; or
— are intended to administer medicines, in which case they are classified as class IIb.”we consider
CLIN-r+ Recommendations
There is a big difference between what a device is intended to achieve (in terms of both clinical endpoints and physiological mechanisms to get there); how a device is technically intended to operate in order to achieve that; and how the user is expected to behave to facilitate all that. These are all things regulators, manufacturers and users need to know and understand.
Your intended purpose and intended use statements are integral to getting your medical device to market. These statements help ensure your device meets regulation when used properly.
Focusing early on regulation will make it easier to design and write the right statements, and often makes it easier to market the device further down the road.
We hope the information in this document helps you assess and manage the impact that Intended Purpose and Intended Use will inevitably have on medical device development and marketing strategy under EU MDR. It can be a minefield, and having an external expert such as Clin-r+ can be an invaluable addition to your Medical Device armoury. Should you have any questions or need professional assistance, CLIN-r+ has a wealth of experience to call upon. Get in touch!




