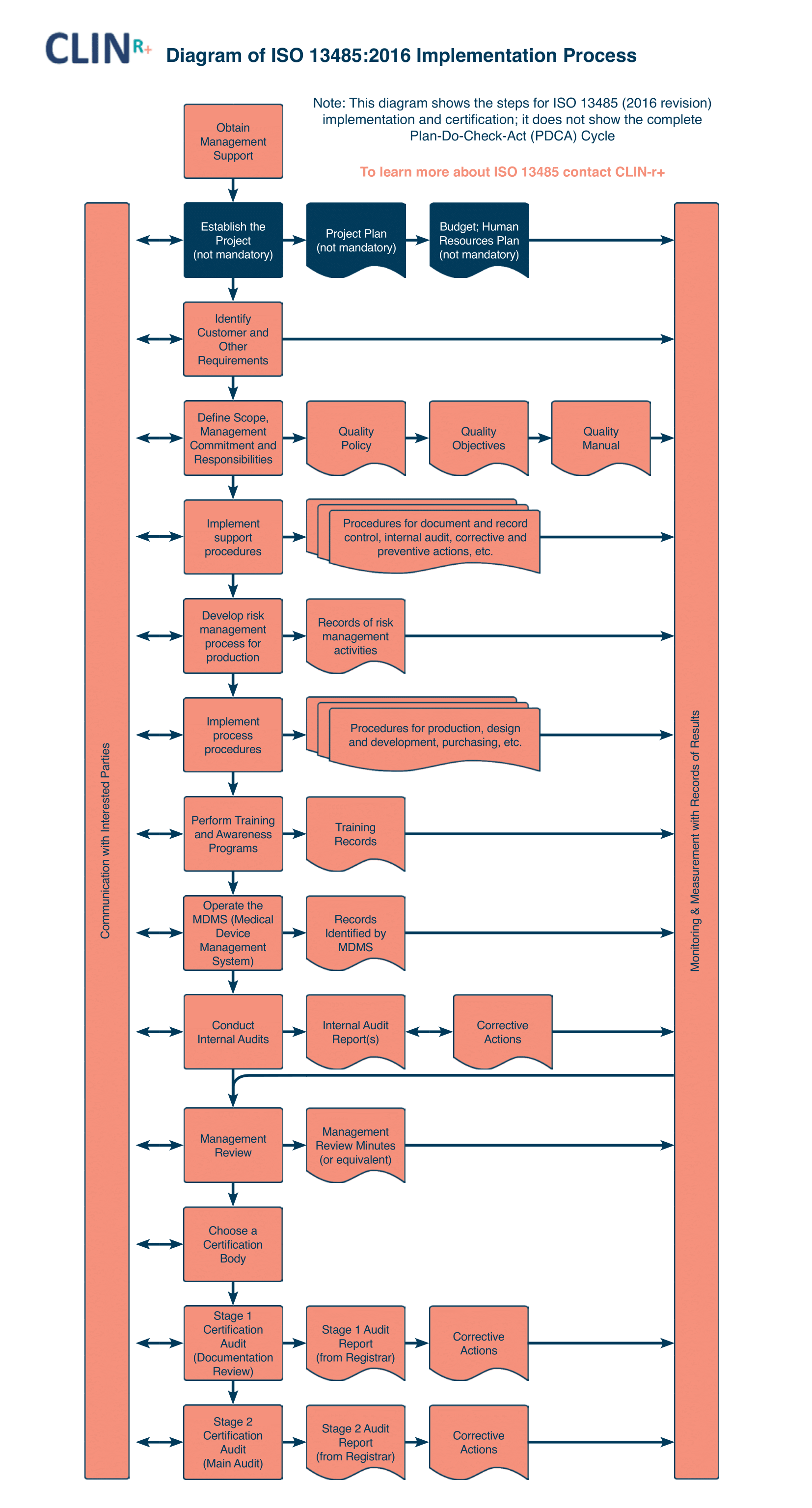
Managing design of a Medical Device
Design & development of MDs according to ISO 13485:2016
The new Medical Device Regulation (EU MDR 2017/745) has introduced many new and significantly updated processes. If a manufacturer sells medical devices in the EU, they must incorporate these changes into their Quality Management System (QMS). These procedures go above and beyond the current ISO 13485:2016 and Medical Device Single Audit Program requirements (MDSAP). The table below shows a high-level comparison of ISO 13485:2016 and the associated MDR requirements. These have an effect on managing design of a medical device.
ISO 13485:2016 | MDR |
| 4.2 Documentation Requirements |
|
| 5. Management Responsibility |
|
| 7.3 Design and Development |
|
7.5.8 Identification 7.5.9 Traceability |
|
| 8. Measurement, Analysis, and Improvement |
|
What are the requirements?
Article 10 (General obligations of manufacturers) and Chapter 1: Quality Management System of ANNEX IX of the MDR lays out the new QMS requirements. They scattered additional QMS requirements throughout the MDR articles and annexes. The MDR adds to Article 10, ISO 13485:2016, and the MDSAP by introducing new requirements. We must analyse these requirements to understand their interdependence and impact on key QMS processes.
Planning: Design and Development
As outlined in ISO 13485 or 21 CFR 820.30, a typical design and development process cycle will include many sequential design stages or phases. These phases typically include Design and Development Planning, Inputs, Outputs, Verification and Validation, Commercialization, Post-Market and Design Changes. Manufacturers should be aware that the MDR does not change the current design control structure in terms of stages or phases. The revised MDR requirements are expected to be integrated into the design control cycle, which begins during planning and continues throughout the design cycle, including the post-market phase.
MDR Annex IX, 2.2 (c) states that –
“The procedures and techniques for monitoring, verifying, validating and controlling the design of the devices and the corresponding documentation as well as the data and records arising from those procedures and techniques shall specifically cover: the strategy for regulatory compliance including processes for identification of relevant legal requirements, qualification, classification, handling of equivalence, choice of and compliance with conformity assessment procedures”.
With that in mind, the design and development process must consider not only the markets in which the device will be sold, compliance with industry and device-specific regulations, and device classification, but also all aspects of the regulatory compliance strategy. Device classification, as modified by Annex VIII of the MDR, and handling of equivalence, as required by Part A of Annex XIV, are included in the strategy and regulatory compliance. These factors could have an impact on product launch timelines, as well as regulatory implications.
Inputs, Outputs, Verification and Validation
When managing design of a medical device, as part of the design and development process, Annex IX, 2.2(c) requires that –
“Identification of applicable General Safety and Performance Requirements (GSPR) and solutions fulfil those requirements, taking applicable Common Specifications and, where opted for, harmonised standards or other adequate solutions into account”.
Many manufacturers currently complete the Essential Requirements Checklist (Annex I) of the Medical Device Directive (MDD) just before submitting a technical file for CE marking. This practice will change, as Annex I (GSPRs) of the MDR contains both significantly updated and completely new requirements that were not included in the MDD. The GSPRs, in conjunction with the applicable Common Specifications and Harmonized Standards, will impose new device requirements and specifications.
As part of the design and development inputs, outputs, verification, and validation phases, these changes must be addressed. Compiling data for CE mark submission will no longer be sufficient. Below are just a few of the significantly updated requirements in Annex I of the MDR.
- GSPR 10.4 (Hazardous substances: CMR: carcinogenic, mutagenic or toxic to reproduction, endocrine disrupting substances)
- GSPR 12 (Devices incorporating a substance considered a medicinal product and devices composed of substances or combinations of substances absorbed by or locally dispersed in the human body), GSPR 17 (Electronic Programable Systems)
- GSPR 22 (Devices intended for use by lay persons)
- GSPR 23 (Label and instructions for use)
It is required that the clinical evaluation process begin during the development of a medical device, as outlined in the MEDDEV 2.7/1 Rev. 4: A guide for manufacturers and notified bodies under directives 93/42/EEC and 90/385/EEC. Furthermore, clinical evaluation and risk management should guide premarket research and development to define the needs for clinical safety and clinical performance of the device. This requires the manufacturer to evaluate any available clinical data, determine equivalence, define data gaps for the device under consideration, and determine whether those gaps require clinical investigations.
The Importance of Post Market
As previously mentioned, Annex IX 2.2(c) of the MDR requires that the clinical evaluation, pursuant to Article 61 and Annex XIV, including post-market clinical follow-up, be covered in the design and development process.
The MDR adopts the same requirements as the MEDDEV 2.7/1 Rev. 4, from a documentation standpoint, with more specific contents included in the Clinical Evaluation Plan (CEP) and Clinical Evaluation Report (CER). Requirements for biological equivalence mandate that materials used have similar release characteristics of substances, including degradation products and leachables. As part of the clinical evaluation documentation, the MDR also introduces two new terms: “Clinical Development Plan” (CDP) and “Summary of Safety and Clinical Performance” (SSCP). Only Class III and implantable devices are covered by the SSCP. Therefore, in the design and development process, the handling of equivalence, potential needs for clinical investigation, as well as the planning and creation of clinical evaluation documentation must all be considered. Throughout the design and development cycle, as well as in the post-market phases, planning, gathering, and evaluating data is required.
 Another process that is interdependent with design and development is post-market surveillance (PMS). Previously, Article 2(60) of the MDR contained a clear definition of PMS, as well as detailed requirements to be met in Articles 83-86 and Annex III. As per the MDD and ISO 14971: Medical devices — Application of risk management to medical devices, planning for Post Market Surveillance could be initiated after the device is placed on the market.
Another process that is interdependent with design and development is post-market surveillance (PMS). Previously, Article 2(60) of the MDR contained a clear definition of PMS, as well as detailed requirements to be met in Articles 83-86 and Annex III. As per the MDD and ISO 14971: Medical devices — Application of risk management to medical devices, planning for Post Market Surveillance could be initiated after the device is placed on the market.
PMS is now part of the technical documentation, since it is now part of the MDR (Annex III).
Therefore, the manufacturer must create the PMS before receiving the CE mark and placing the device on the market under the MDR. This includes activities documented in a PMS and a PMCF plan to be implemented once the device is on the market.
What does an MDR-compliant design & development process look like?
The table below summarises the preceding discussion and lists additional design records that manufacturers must create during the design development phases when managing design of a medical device.
Design Phase | Design and Development Records Required by MDR |
Planning |
|
Inputs |
|
Outputs | |
Verification and Validation | |
Post Market |
CLIN-r+ top tips on being compliant:
A typical question asked by manufacturers is whether the technical documentation for legacy devices needs to be remediated. The answer is: Yes. The assumption is that manufacturers start with a blank page and document all objective evidence needed to meet Annex II and III requirements and comply with the applicable General Safety and Performance Requirements (GSPRs).
Another typical question is whether design control records, such as the design history file (DHF), also need to be remediated. Again, the answer is: Yes. There are likely to be new design inputs and subsequent outputs, validation and verification records, as applicable, during the remediation of the technical documentation and addressing the additional requirements of the GSPRs. Therefore, design changes to existing DHFs should be captured as part of impacting design records. If the significantly revised or new GSPRs do not impose additional requirements on the device being remedied, the labelling (labels, IFUs) will almost certainly be updated due to GSPR 23.
We hope this document has answered your questions. Should you have any other questions or need professional assistance, CLIN-r+ have a wealth of experience in managing design and development for Medical Devices. Get in touch!