UDI Beginners Guide
Unique Device Identification (EU MDR and IVDR)
One of the new features introduced by the new EU MDR 2017/745 and IVDR 2017/746 is the UDI, also known as a “Unique Identification Number” in Europe (similar to the UIN used in the USA).
This new component, which will greatly aid the traceability of devices in Europe, originated from the IMDRF guidance published in 2013. And whilst it is nothing new for the medical device industry, as it has been a necessity for goods delivered to the US market for many years, there will be work to do for manufacturers not selling devices in the USA.
The Unique Device Identification (UDI) number is crucial, as it will be required for product tracking. We use the EUDAMED database for this purpose. However, EUDAMED has not been fully set up (at the time of writing), and it is unknown when it will be.

What is a UDI or Unique Device Identification?
Simply put, a UDI is a code that designates a certain product. There are 2 sections to the UDI that are on your goods. These are:
- The UDI-DI – The device identifier. It designates a particular device in your portfolio. This is the static portion of the UDI number. It stays the same throughout the entire identical product.
- The UDI-PI – The production identifier. It is the UDI’s dynamic component. The lot number, serial number, manufacturing date, and expiration date are all disclosed.
Let’s look at an example of a UDI. You can see an example of a linear bar code in the example below.

In addition to the machine-readable barcode, there must be a component that can be read by a human. Known as HRI, or Human Readable Interpretation. The UDI-DI is always the number following the (01). Every time the number 01 appears in brackets, think of this as a placeholder for the UDI-DI.
After the (01) placeholder, you can see the UDI-PI portion which can vary depending on the product’s production characteristics. Some of the numbers in the brackets on the UDI-PI component will be recognisable. Each of these numbers conveys specific details about the item:
- (10) is for the lot number.
- (11) is for the production date.
- (17) defines the expiration date.
- (21) is the serial number.
This means that every new batch or updated expiration date, will have all of these details change, making the code dynamic.
What is the Basic UDI-DI?
Annex VI Part C of the MDR 2017/745 and IVDR 2017/746 both provide a general explanation of what a Basic UDI-DI is. The MDCG 2018-1 Draft Guidance on Basic UDI-DI is another tool you can use, which offers some additional information, which is summarised below.
The Basic UDI-DI is an identification number that applies to a group of products, not a single item. It should be noted that a Basic UDI-DI has no supply chain value, but serves for administrative purposes.
One Basic UDI-DI is for devices within the same category:
- Intended purpose
- Risk class
- Essential design
- Manufacturing characteristic
The Basic UDI-DI will appear on your:
- Certificates (Notified Bodies)
- Declaration of conformity
- Technical Documentation
- Summary of safety and clinical performance
- Certificate of free sale (Article 60(1) of MDR)
It may surprise you that the Basic UDI-DI does not appear on the product packing. It is a number that your customers won’t see and, as previously stated, serves for administrative purposes only. Additionally, you may have one or more UDI-DIs beneath this Basic UDI-DI (specific product identification).
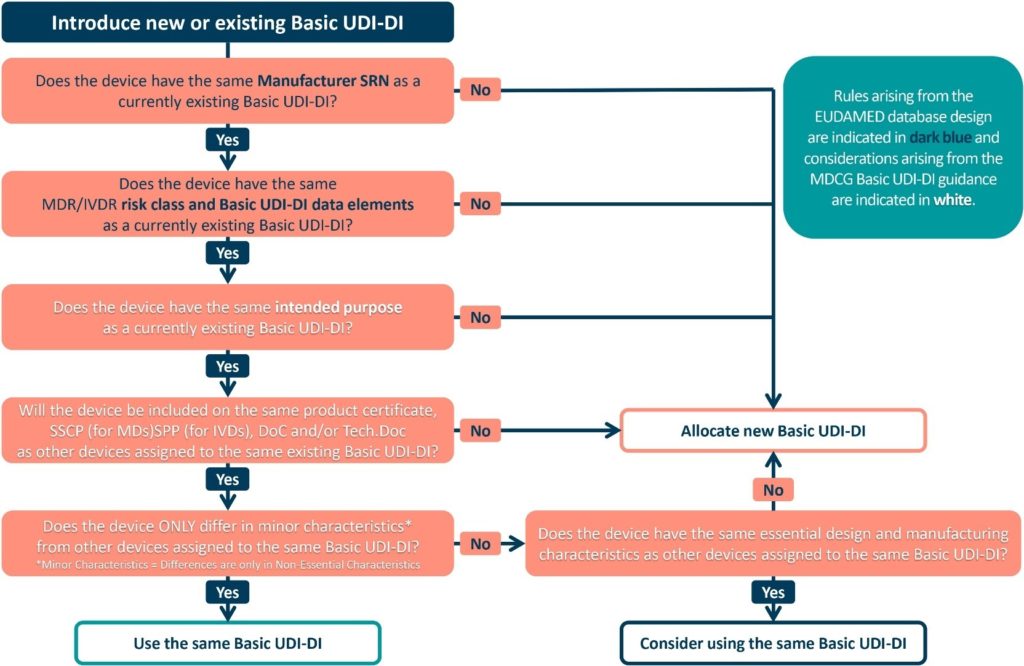
Decision Tree (Medtech Europe)
To assist you in grouping your products, please see the decision tree below. This decision tree takes into account the considerations arising from the EUDAMED database design (indicated in dark blue) and the MDCG Basic UDI-DI guidance (indicated in white). By following its guidance, you can successfully assign a Basic UDI-DI to your products.
Where can I get the Basic UDI-DI and in what format?
Generally, an official designated entity provides the Unique Device Identification. The selected entity should give you all the resources you need to build your Basic UDI-DI. The Commission should do the designation if you are looking at Article 24(2).
The MDCG 2019-1 offers several guidelines for issuing entities regarding Basic UDI-DIs. The Basic UDI-DI format should follow the examples shown in this document as closely as possible.
- Maximum of 25 characters, as this is the maximum length of the UDI-DI.
- The organisation that will provide you the UDI code should also implement a check digit/character.
- The organisation should also provide an algorithm (to the Commission and the manufacturer) to verify the code.
What is the UDI-DI (as opposed to the Basic UDI-DI)?
You now have your Basic UDI-DI for your range of products. For each one, you also require a UDI-DI.
Let’s take hip implants as an example. Your hip implant group does indeed have a Basic UDI-DI. However, there are three categories of hip implants within this group. “COa,” “COb,” and “COc.”
As a result, each hip implant will have the same Basic UDI-DI and they will also have a single UDI-DI.
- COa – UDI-DI 1
- COb – UDI-DI 2
- COc – UDI-DI 3
The image below illustrates the 3 hip implants, all with the Basic UDI-DI CO, and their own individual UDI-DIs too.
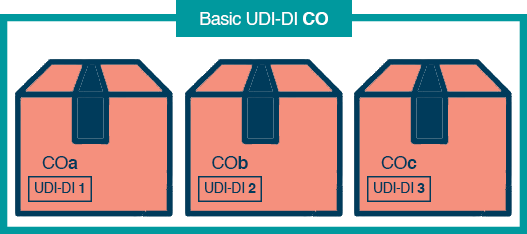
UDI-DI CO
For each Device (Name or trade brand)
Basic UDI-DI CO
For a group of same products. Visible on:
- CE certificate.
- Declaration of Conformity.
- Technical Documentation.
- Summary of Safety and Clinical Performance.
UDI-DI in case of changes to your product
The UDI-DI you were given for your products could be subject to change if you make any alterations to your products.
A new UDI-DI will therefore be necessary if you modify:
- Name or trade name.
- Device version or model.
- Labelled as single use.
- Packaged sterile.
- Need for sterilisation before use.
- The number of devices in a package.
- Critical warning or contra-indication.
- CMR / Endocrine disruptive.
- Colour.
- Language.
This can result in a different UDI-DI for the same product provided in a different quantity of packaging or in a different state (sterile, non-sterile, single use). If we return to our hip implants example, and produce multiple iterations of the COa product:
- Sterile version.
- To be sterilised version.
- Packaged per 10 pieces.
As a result, each hip implant will have the same basic UDI-DI, and they will also have a single UDI-DI.

UDI-DI CO
You should have a UDI-DI for each version of your product, even if each unit is exactly the same.
Example: Hip Implants “a” delivered sterile will have a different UDI-DI than the same hip implants packaged per 10 pieces.
You will therefore want a UDI-DI for each version of your product, which will be linked to a single Basic UDI-DI. In our hip implant example, each of the three products with their 3 versions of each product would have nine UDI-DIs, which would all be linked to a single Basic UDI-DI. It should be noted that there would still only be one Technical File, which would include all the products.
What is the UDI-PI?
UDI-PI stands for Unique Device Identification – Production Identifier.
Let’s assume you are a manufacturer of Product A Version 4 in 2022.
- On April 18th, you produce 5 pieces.
- Then on April 19th, you produce another batch of the exact same product with 3 pieces.
- Then on April 20th, you produce a further 4 pieces.
So, we would end up with the situation below:
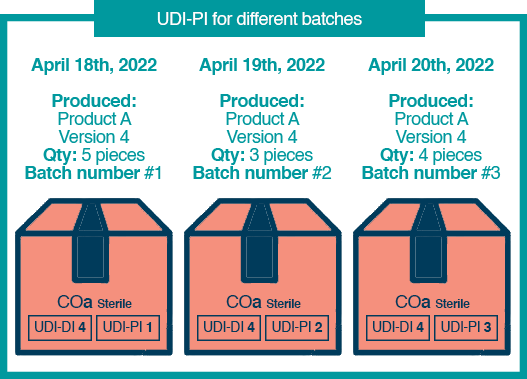
As you can see from the illustration, the product keeps the same UDI-DI regardless of the day the manufacturer produced it. However, the UDI-PI has changed in relation to production (the batch number).
The UDI-PI changes to reflect various parameters, including Date of Manufacture, Batch Number, and Expiry Date, to name a few.
UDI-carrier
The UDI-carrier is the format in which the UDI can be shown. Additionally, it has two components, the AIDC and the HRI:
- Automatic Identification and Data Capture (AIDC) – is a technology used to automatically capture data. AIDC technologies include bar codes, smart cards, biometrics and RFID (Radio Frequency Identification).
- Human Readable Interpretation (HRI) – is a legible interpretation of the data characters encoded in the UDI carrier. It´s important in case there is no automatic system to read the code.
The UDI-carrier, therefore, should initially have a section that is read automatically, like a bar code. Followed by a part that humans can read, like numeric codes.
Legacy devices
From May 26th, 2021, to May 26th, 2024, there will be a transitional period during which both MDD and MDR items will be on the market. However, MDD products will also need to follow some MDR-specific regulations.
For example, any vigilance reporting should follow the MDR requirements, even if it is an MDD product. However, one of the requirements when reporting a vigilance issue is to identify the product by its UDI number. UDI codes are only required for MDR items, and thus, MDD products would be exempt from this rule. What would be the best way to trace the product on the EUDAMED database? The EU Commission has established guidelines and can be found here.
UDI vs Primary packaging and Secondary packaging
Packaging levels means the various levels of device packaging that contain a defined quantity of devices, such as a carton or case. One area manufacturers often find confusion is whether they require one UDI-DI for the primary packaging and another for the secondary.
The answer is a refreshingly simple “no”. In the case of a bottle, for example, with its own label (primary packaging) and a box that protects the container and contains information about the product inside (secondary packaging). As long as the bottles are of the same batch, i.e., they have the same UDI-PI, then you shouldn’t regard it as having two tiers. In this case, the product’s UDI-DI and UDI-PI are identical. One unit of the product is contained in each supplementary packaging. Consequently, they all continue to function as one unit.
If, however, there are bottles from different batches in a single carton, perhaps two bottles weren’t made on the same day and were packaged later, for example, then the bottles would have a unique UDI-PI. Consequently, there is a second level.
Where should I place the UDI on my product?
There are guidelines on where the manufacturer should display the Unique Device Identification. The user of the product should typically see the code. You must include the UDI carrier on the device label and all upper-level packaging.
However, there are circumstances where this might be different.
For instance, the manufacturer should individually pack and label class I and IIa single-use devices. In that situation, the next higher packaging may be used to place the UDI code, rather than directly on the primary package. These products in separate packages may be contained in this packing in multiples.
You should display the UDI prominently reusable equipment, without exceptions. The FDA approves the AIDC or HRI, and both should be visible.
Where can I register for the UDI?
Typically, you must contact a company that has the right to give you a UDI-DI code. This is the code unique to both your business and your products.
Article 120(12) of MDR 2017/745 it says:
“Until the Commission has designated, pursuant to Article 27(2), issuing entities, GS1, HIBCC and ICCBBA shall be considered to be designated issuing entities.”
The European Commission should designated the businesses permitted to offer this UDI-DI number. This can be challenging, and the number of codes you require depends on your portfolio.
GS1, HIBCC, and ICCBBA are designated issuing entities, which already offer UDI-DI to US manufacturers.
On June 6th, 2019 a Commission Implementing Decision (EU) 2019/939 was issued, highlighting that the IFA GmbH was a new entity on the official list of the designated entities. Perhaps more will appear later, but for now, here is the list of designated issuing entities:
UDI Format guidance
For each of the designated entities listed in the previous section, the European Commission has released certain guidance. Once you have identified the entity that will issue your Unique Device Identification, you can then look at its particular format.
Though the idea behind UDI-DI, UDI-PI, and Basic UDI-DI remains the same throughout, each entity has different letters or numbers.
Below you will find links to useful information regarding the UDI format by issuing entities.
GS1
• Basic UDI-DI Format
• UDI HRI & AIDC Formats
HIBCC
• Basic UDI-DI Format
• UDI HRI & AIDC Formats
UDI for combination products
Combination products often incorporate many regulations. For instance, a product that comprises both a drug and a medical device, or perhaps a drug and cosmetic. However, the product will mostly adhere to a single rule. Depending on the intended use.
If you sell combination products, you must determine whether UDI is applicable to your product. We can see the rule to follow on the MDCG 2019-2.
To summarise, you must adhere to the UDI criteria if your product is a medical device, but also contains a pharmaceutical.
EXAMPLES from the MDCG:
- Catheters coated with heparin or an antibiotic.
- Soft tissue fillers incorporating local anaesthetics.
- Implantable infusion pump.
- Spacer devices for use with metered dose inhalers.
- Bone void filler with an antibiotic.
- Bone void filler with animal growth factors, where the action of the growth factors is demonstrated to be ancillary to the physical filler.
However, if the medical device component of your product is covered by the Medicinal Directive 2001/83/EC, a UDI is not mandatory.
EXAMPLES from MDCG:
- Non-reusable autoinjectors containing a medicinal product as integral part.
- Nebulizers pre-charged with a specific medicinal product.
- Patches for transdermal drug delivery.
- Wound dressings impregnated with an antibiotic, where the primary intended purpose is to administer the antibiotic to the wound.
UDI for standalone software
The only software that can be commercialised alone is in the scope of Annex 6 Part-C paragraph 6 of the EU MDR.
A software Unique Device Identification must have the two pieces, just like other products (UDI-DI and UDI-PI). The UDI-PI should show the manufacturing parameters, and the UDI-DI should show generic software information.
In the case of changes as software is continually maintained, the manufacturer should change only the UDI-PI in the event of minor updates, such as minor bug fixes, usability improvements without safety implications, security patches, or operating efficiency improvements. However, if there are significant changes, a new UDI-DI must be requested. There are 3 cases defined on the MDR where you’ll need to change the UDI-DI number:
- The original performance.
- The safety or intended use of the software.
- the interpretation of data.
All this can include new or modified:
- Algorithms.
- Database structures.
- Operating platform.
- Architecture or new user interfaces.
- Channels for interoperability.
Your UDI number should be listed on the packaging as HRI and AIDC if you deliver your programme on a CD or DVD. The UDI linked to your system level software and the one to your packaging level should be the same. Make the UDI HRI plain-text format accessible on the start-up screen or as a “about” file if your software can be seen on a computer screen, for instance.
EU Unique Device Identification transition period
It will take time to implement the UDI requirements. The time allocated depends on the product’s risk. Therefore, the greater the danger, the quicker you should adopt the UDI requirements.
Below are the implementation dates following the class:
- Class I: 26th May 2025
- Class IIa and IIb: 26th May 2023
- Class III: 26th May 2021
- Implantable devices: 26th May 2021
It should be noted that the European Commission is delaying its implementation by two years for reusable items, such as surgical instruments, because certain products would already be on the market when it is implemented (depends on the class). If the reusable product is class III, then the implementation of UDI has been delayed until 26th May 2023. If it´s classification is class I, then implementation has been delayed until 26th May 2027.
CLIN-r+ recommendations
- Preparing for UDI implementation is no small task. We strongly recommend that you remember the key points summarised below:
- Basic UDI-DI is an administrative code that is solely used to group devices that belong to the same category and to allow authorities to identify the manufacturer of the product. The same organisations that provide you your UDI-DI can also give you that.
- The device identifier (UDI-DI) is a fixed component that you obtain by registering with an authorised entity (GS1, HIBCC, ICCBBA, IFA).
- The UDI-PI (Production Identifier) is a dynamic component associated with the product itself (Lot number, expiration date, serial number, etc.).
This product’s UDI should be accessible via the UDI-carrier, which is how we view the UDI. It should be visible in two formats:
- Automatic Identification and Data Capture (AIDC). We use the AIDC for any automatic device that can scan the code.
- Human-readable Interpretation (HRI). The HRI must be legible (to be read by humans).
- For combination products. If your product is regulated as a Medical Device, you need to apply UDI. But if your product is regulated as a Medicinal Product that incorporates a Medical Device, then it’s not mandatory to apply UDI.
Clin-r+ highly recommends you use the following webpage created by the EU Commission to better understand UDIs.
We hope the information in this document helps you to implement your UDIs. Should you have any questions or need professional assistance, CLIN-r+ has a wealth of experience to call upon. Get in touch!





