Clinical Evaluation Reports & Review

What is Clinical Evaluation?
Clinical Evaluation is a proactive, continuous process. It gathers data and evidence for the safety and performance of your medical device. The Clinical Evaluation is a key workflow to and must demonstrate that a device is safe and performs as intended.
Clinical evaluation is the responsibility of the manufacturer, and the clinical evaluation plan and report are critical elements of the technical documentation for a medical device.
What do you need to be compliant?
We know that the new regulations and all that clinical evaluation now entails can be problematic. Clinical Evaluation has evolved from a simple process and report to a detailed justification of your medical device and critical review of its safety and performance. Data from trials and comprehensive literature searches must support the evaluation.
Your clinical evaluation report should be updated every year to every 5 years depending on the device classification. Manufacturers must ensure clinical evaluation is aligned with all the other technical documents for each device. This provides essential consistency and also gives your Notified Body confidence in your evidence and device documentation.
How can Clin-r+ help?
Clinical evaluations are where CLIN-r+ sets itself apart and will help your product come alive in its contribution to improving health outcomes. Not only is a strong CER needed for EU MDR but is can also help you post market. Our Clinical Evaluation approach has verified audit success. Whether you are starting the process with a brand new device or are established in the market, our services excel in this area.
This has been further enhanced with agile live project management to assure a six week turnaround with highly skilled medical writers and clinicians reviewing your SoTA. This ensures correct identification of safety and performance endpoints and thorough examination of the device’s safety and performance characteristics.
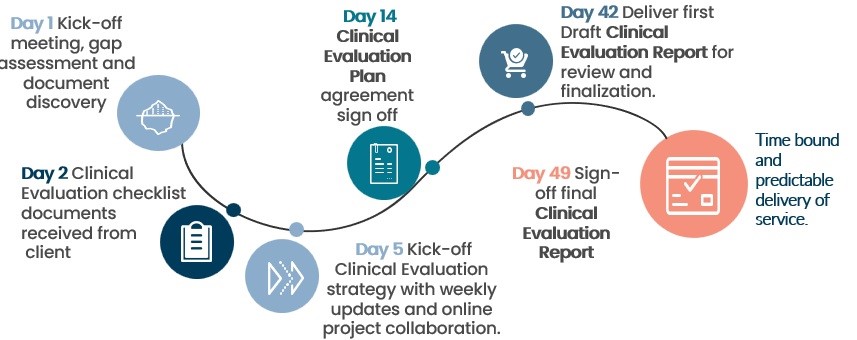
Our experienced CER team will not only assist you to identify problem upstream, but also advise on solutions, best practices deployed by the MedTech industry, and efficient workflows to overcome problems later on.
Need more details?
We are here to assist. Contact us, set up a meeting, join our mailing list or follow us on LinkedIn.
See our ‘Ways of Working together’ page and our ‘Case studies how we have helped manufacturers not just comply but tame their regulatory documentation and resource needs.
You can also check out these white papers on our MedTech Academy:



