Safety Assurance of Your Medical Device
Biocompatibility safety
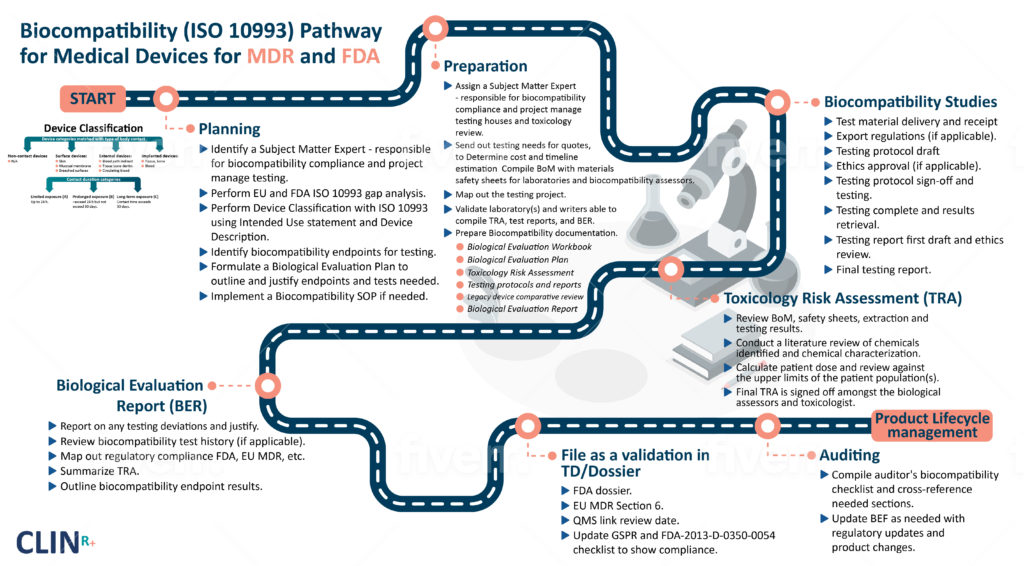
Biocompatibility
Evaluations
Biocompatibility testing provides safety assurance for Medical Devices. Our testing and reporting are specialised for Medical Devices.
What is the benefit?
Work with a consulting firm that is an extension of your business. We bring our experienced Toxicologists to ensure your biocompatibility evaluation testing investment, in cost and time, is maximised.
Reduce the likelihood and number of deficiency findings during your CE assessment of your Device BEF. Avoid a redo of expensive and timeconsuming tests.
Create an extension to your business and have Materials and Toxicology expertise on demand to keep abreast with consumer needs and the dynamic MedTech regulations.
Are you experiencing the current problems?
If you have these problems we can help:

You don’t have the in-house resources or expertise to do an Biological Evaluations Plans to meet FDA/EU MDR.

Your previous lab created biocompatibility reports and now you have deficiency findings

You are uncertain if your current Biocompatibility documents will meet the FDA or EU MDR requirements

You do not have the inhouse skills/staff/resources to do Biocompatibility workflow.
Is your Biocompatibility Evaluation File (BEF) workflow EU MDR ready?
Unsure?
Why not book a free 15 min assessment with an experienced consultant to pre-assess your auditors (NB) response. You will receive a personalised score card to guide your corrective action plan.
Biocompatibility Evaluations (BE)
production timelines
The timeline of each medical video project differs, however, we do have averages to guide you on how long before your project is delivered.
- Materials worksheet
- BE Plan
- Chemical characterisation
- Toxicological Risk Assessment
- Laboratory testing
- BE Reports
- 1 week
- 2 - 5 weeks
- 2 weeks
- 2 - 4 weeks
- 4 - 24 weeks
- 4 - 24 weeks
Our Biocompaibility Evaluations are an end-to-end service, you give us the product we do the rest:
BiocompatIbility plans, assessments, and evaluation reports:
- BEP
- TRA
- Chemical characterisation
- BER
Local effects
testing:
- Cytotoxicity
- Sensitisation
- Irritation
Systemic effects
Testing
- Mediated Pyrogenicy
- Toxicity
- Implantation
- Hemocompatability
Next steps to get CLIN-r+ support:
01.
Book a free 30 minute introduction call
for a rapid gap analysis.
02.
Receive a personalised
gap assessment and detailed project proposal.
03.
Onboard the CLIN-r+ team and spend time on daily business,
where it matters.
How can Clin-r+ help?
Below is more information and case studies how our MedTech clients benefited from our Biocompatibility Evaluations support. See what CLIN-r+ Toxicology team can do for your project:
Not ready to book a call?
No problem, have a look at a few of our articles on Biological Evaluation Workflow: