IVD Performance Evaluation Planning & Report (PER)
Requirements in the IVDR 2017/746
Has a European Notified Body scrutinised your technical documentation? Except for those selling high-risk IVDs in Europe, the likelihood is no, it hasn’t.
Well, that is about to change. Due to Europe’s new In Vitro Diagnostic Regulation (IVDR 2017/746). They will impose stringent new requirements on many IVD manufacturers pursuing CE marking for their devices.
Going forward, a European Notified Body designated to the IVDR will be responsible for the approval of the quality management system and technical documentation of an estimated 85-90% of all IVDs seeking CE Marking certification in Europe. This differs greatly from Annex III of the In Vitro Diagnostic Directive (IVDD), which, although it called for performance evaluation data, focused exclusively on analytical performance. The Notified Bodies neither unequivocally stated nor regularly scrutinised this data requirement.
What will these new requirements by the IVDR entail?
This is undoubtedly the question you wish to jump to, and rightly so. Nevertheless, it would be well within your interests to first take a moment to understand your performance evaluation obligations. Making sure you have the appropriate repository of documents handy is a good starting point.
Have you already downloaded a copy of the IVDR? It is best accessed on site online version instead, because of its linkable table of contents. You won’t regret it! Chapter VI and Annex XIII are the most pertinent sections here. Beyond those, you should download these documents to your desktop:
- GHTF SG5/N6:2012 – Clinical evidence for IVDs: Key definitions and concepts
- GHTF SG5/N7:2012 – Clinical evidence for IVDs: Scientific Validity Determination and Performance Evaluation
- GHTF SG5/N8:2012 – Clinical evidence for IVDs – Clinical performance studies
- ISO 20916:2019 – Clinical performance studies using specimens from human subjects
- MDCG 2022-2 – Guidance on general principles of clinical evidence for IVDs
Finally, as the IVDs classifications (A, B, C, D) are completely new, you would also benefit from downloading a copy of this IVD classification guidance document.

Plan for success… Performance Evaluation Process and Planning
Preventing false positives and false negatives, both of which could lead to detrimental physical and psychological impacts for patients, is among the most important reasons for performance evaluation.
While the temptation to dive into the nitty gritty of the report is there, the writing of your Standard Operating Procedure (SOP) and creating your comprehensive Performance Evaluation Plan (PEP) as part of the planning requirements, are critical to the report’s success. In fact, omitting the SOP would cause immense problems further down the line, since Article 56(3) of the IVDR mandates a process established and implemented.
What if your device already has US FDA 510(k) clearance? Though this may be the case of many, due to the strictness of the EU IVDR regarding the demonstration of clinical benefits, it is highly probable that you will need to generate (and analyse) more data than you have at this moment! Furthermore, the opinions of clinical benefits related to IVD medical devices will vary considerably between the European regulators and US FDA.
 Although no two performance evaluation plans are the same, there are certain elements that should be included in all of them without fail. Including, though not limited to:
Although no two performance evaluation plans are the same, there are certain elements that should be included in all of them without fail. Including, though not limited to:
- Your performance evaluation supports the General Safety and Performance Requirements (GSPR).
- Intended use or purpose of the device.
- Indications for use, limitations, and contra-indications.
- Performance specifications of the IVD.
- A description of the current state of the art.
- Benefit-risk ratio parameters.
- Intended target patient groups.
- Analyte or marker to be used.
- Foundation for underlying software decision making (if applicable).
Dedicate sufficient time to prepare this plan, and it will pay dividends when you complete the performance evaluation, saving you many more hours than those you invested during the planning stage. The possibility of your product’s risk assessment and risk management processes being altered by your “research” is always something to bear in mind. Moreover, it is advisable to consider whether you have the internal resources to administer the numerous aspects of the performance evaluation. Otherwise, bringing in outside expertise may be something that needs consideration.
Steps in the Performance Evaluation Process
Proving Scientific Validity of your IVD
Starting to prove scientific validity early in the life cycle of your IVD is paramount when bringing a new product to the market. A key method for achieving this is to use the relevant information on the scientific validity of other devices measuring equivalent analyte or marker. It goes without saying that we can perform a detailed literature search. However, if adequate evidence is lacking, a proof of concept, pre-clinical studies, or clinical performance studies will be required. Your scientific validity report will summarise your findings and become part of your performance evaluation report. For further information, please see IVDR Annex XIII, Part A.
Evaluating Analytical Performance of your IVD
IVDR Annex I, Section 9.1 comprehensively describes the performance characteristics that must be measured. Said characteristics include sensitivity, specificity, repeatability, reproducibility, predictive value, and more. We must maintain records of these performance characteristics throughout the life of the device. You should carefully plan sampling, as the ability to create reproducible results is critical. The Analytical Performance Report will summarise the outcome of your analytical performance.
Evaluating Clinical Performance of your IVD
A further requirement is to conduct analysis of clinical performance. Measuring the diagnostic accuracy of your device is the focus here, and in most cases, retrospective samples will be necessary rather than a prospective clinical study. If you (either through choice or obligation) need to rely on peer-reviewed literature, ensure your search criteria and methods have been meticulously documented. Furthermore, your Notified Body should be consulted early on if there is any doubt about whether a clinical study should be conducted, type/number of samples, or how a study contributes to performance evaluation. A protocol should be written before conducting any study, and that is where ISO 20916:2019, jointly with the requirements outlined in Annex I, 9.1, becomes very useful. Reasoning behind collection methods should be meticulously documented.
It is also necessary to examine claimed shelf life, shipping stability, and in-use stability, as dictated by Annex II, 6.3 of the IVDR. And, you guessed it, further reports are required to be generated for that too. On a positive note, you’ll be a seasoned report writer by the time your NB audit comes around!
What is an IVD Performance Evaluation Report (PER)?
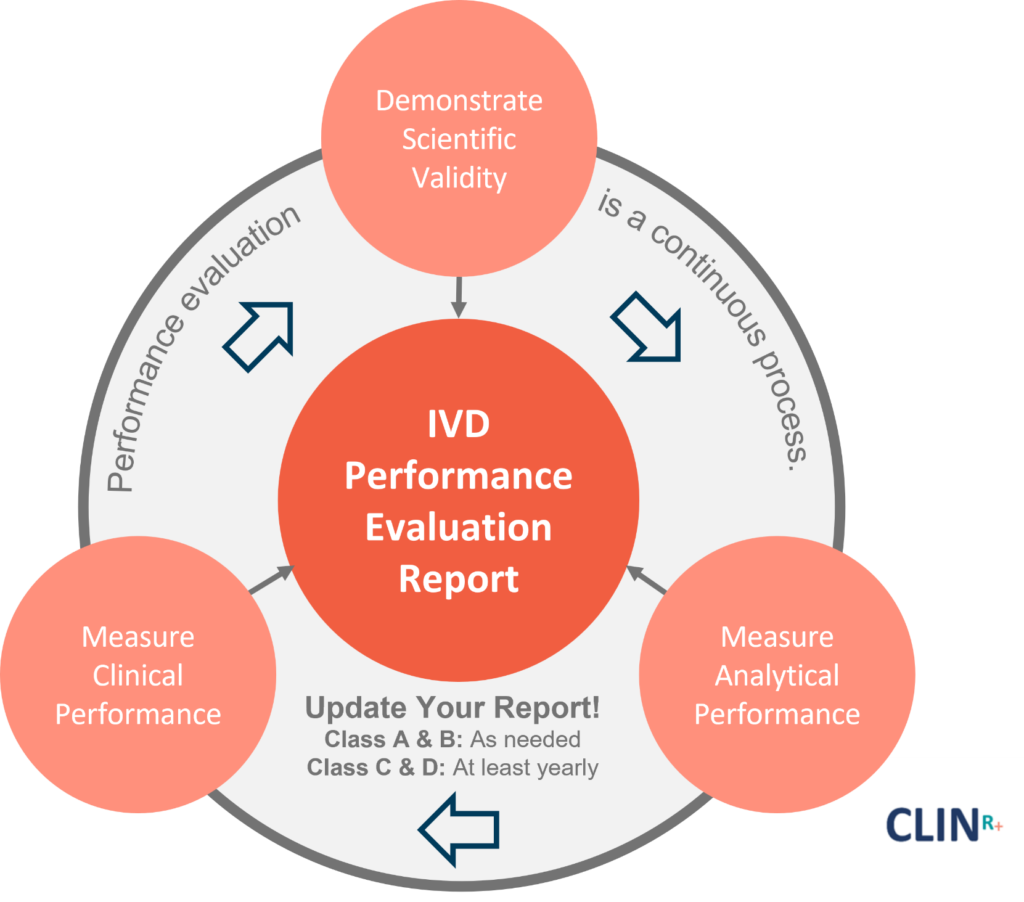
Now that you have a sturdy plan to follow, analyses of scientific validity, analytic, and clinical performance all ticked off your checklist. Assembly of the final performance evaluation report awaits you. This is where you will reap the benefits of all the hours invested in the planning process.
The key requirements for your PER are set out in Annex XIII, Part A, 1.3.2 of the IVDR, including:
- Classification of the IVD, taking into account safety and performance characteristics.
- Intended purpose of your device and performance or safety claims.
- Technology on which your IVD is based.
- Justifying the approach you have taken for gathering clinical evidence.
- Detailing your literature search methodology, protocol, and report.
- Extent of scientific validity plus all analytical and clinical performance data evaluated.
- Evaluation of clinical evidence compared to the current state of the art.
- Results of Post Market Performance Follow-up (PMPF) studies.
Class A & B devices can have their PERs updated as needed. However, the same reports for Class C & D devices can also be updated as needed, but must be updated at least yearly.
Evaluation of IVD performance is not a one-time project, and a continued monitoring of your product performance throughout its life cycle is mandated by Annex XIII, Part B of the IVDR.
Your completed performance evaluation will be connected to your post-market surveillance system via the Post-market Performance Follow-up (PMPF), which must also be updated throughout the life cycle of the device.
Want to learn more?
The IVDR is a completely new regulation, and should therefore you shouldn’t approach it as simply the IVDD with a new lick of paint. CLIN-R+ offers several solutions to help you make sense of IVDR. Firstly, if you are a newcomer to the regulation, our in-depth IVDR overview training classes are worth checking out. For seasoned auditors, our IVDR auditor training will certainly be of interest to you. Lastly, we offer assistance with gap analysis assessment and getting your technical documentation into shape. No task too big, and indeed no task too small.
To learn more about CLIN-r+ CE Technical File preparation services for medical devices and IVD manufacturers, get in touch!

