Clinical And Technical Documentation
What is Clinical and Technical Documentation?
All medical device approvals and audits of Quality Management Systems (QMS) require compliant Technical Documentation (often referred to as a Technical File). Compiling your Technical Documentation for EU MDR/IVDR can be daunting.
The Technical Documentation is the full set of documents that a device manufacturer must compile and provide to all Regulatory Authorities and Notified Bodies. It’s a prerequisite for the majority of conformity assessments. The MDR/IVDR has increased requirements and set out what is now expected of this documentation.
What do you need to be compliant?
It can be overwhelming reading the regulations, MDR, IVDR, IMDRF, STED, GSPR, MEDDEV 2.7.1rev4, etc.
There is an easier way. Leverage CLIN-r+ experience in creating EU compliant Technical Documents that not only gain you your CE mark but also make it easy to maintain your paperwork for audits.
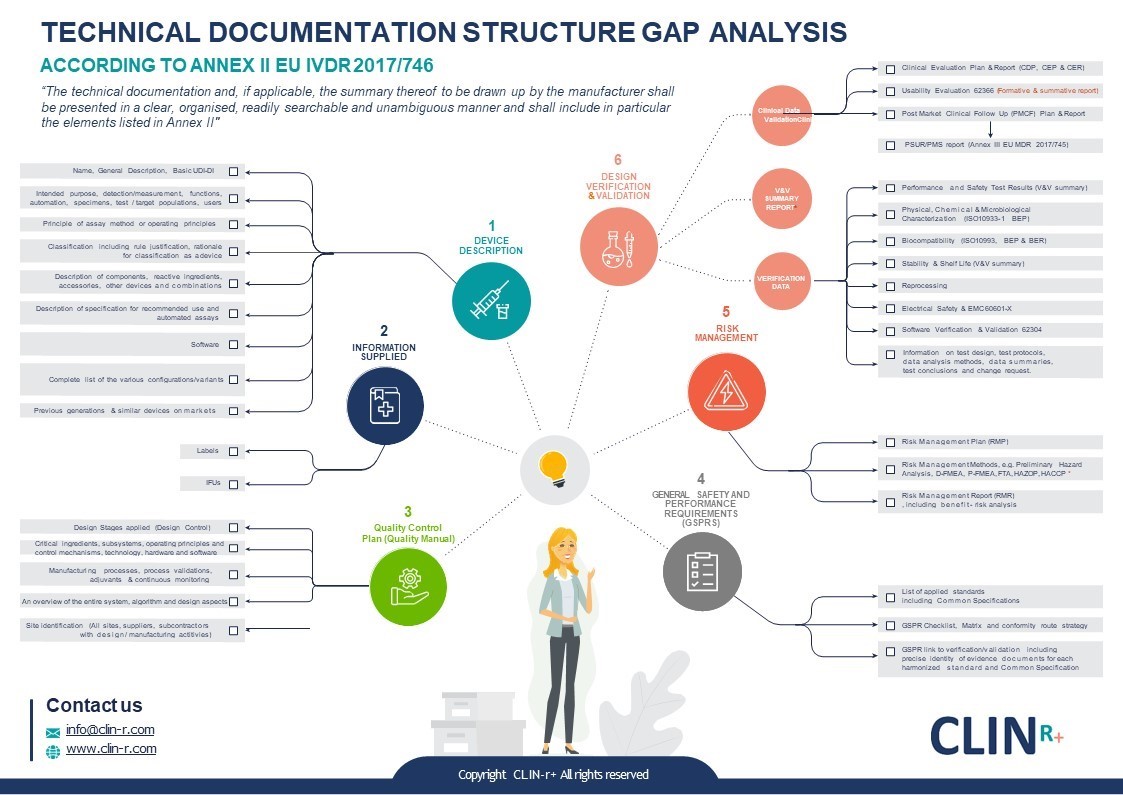
How can Clin-r+ help?
Our technical documents are tailored to our clients and are always produced to the highest standards. Our regulatory experts will also conduct a gap assessment so it’s clear what needs to be done to be compliant. We can ensure that your structure is correct according to EU regulations and contains what your Notified Body expects to see.
We can be involved in compiling individual documents or supporting you with all aspects of the Technical Documentation.
Clin-r+ has created hundreds of technical documents across all device classes. Our international team have the clinical and regulatory knowledge to ensure your documents meet the right requirements. We align with your Notified Bodies processes and checklists to make sure you get it right, first time!
Need more details?
We are here to assist. Contact us, set up a meeting, join our mailing list or follow us on LinkedIn.
You can also check out these white papers on our MedTech Academy:
- How to Structure your Medical Device Technical Document
- Medical Device Design
- Instructions for Use (IFU)
- Labels for Medical Devices
- Unique Device Identification (UDI)
- Risk Management
- Post Market Surveillance
- Clinical Evaluation for Medical Devices
