The Impact of “Intended Purpose”
on Medical Device development & marketing strategy under EU MDR
In recent years, the regulatory environment in Europe has been transforming. This has had a profound impact on medical devices validations and marketing claims. This might not be clear at first when reading rules or guidance. But a closer look at the impact of “Intended Purpose” is an area that needs careful review. Intended Purpose states what the device is, its use, and all the requirements in the MDR and IVDR that are underpinned by it. If your “Intended Purpose” is wrong, it’s all downhill from there from a regulatory point of view.
What is known about ‘‘Intended Purpose’’ under EU MDR
The EU MDR frequently uses ‘Intended Purpose’, citing it 87 times in the regulations. It serves as the cornerstone for demonstrating that your devices comply. Reviewing the ‘Intended Purpose’ sections reveals that this is the starting point that establishes it as medical equipment and justifies its positioning on the market. It also has many connections to other crucial concepts for conformance.
Article 2 of the MDR, which establishes the terms for conformity, is worth dedicating some time to. However, remember that this is not a complete list of terms used in the regulations. To ensure all your language and terminology are consistent, you should also check the MDCG, MEDDEV, and appropriate standards (such as usability).
What is the problem - implications if the ‘‘Intended Purpose’’ isn’t aligned with the CDP or Business Development Plan
Upon beginning to put together your Technical Documentation, or when you’re working on your transition from the MDD “Technical File” to MDR “Technical Documentation”, it becomes clear how difficult it is to align inter-department information. Feeling like your business development plans have ended as soon as you started your regulatory submission? That might be the unfortunate reality.
If your business plan’s “Intended Purpose” isn’t marketing-focused, reaching your target market may be challenging. Any changes to the “‘Intended Purpose’” are considered “substantial” as stated in Article 120 and MDCG 2020-3 (see Figure I). Somewhat predictably, fixing this could be both expensive and resource intensive.
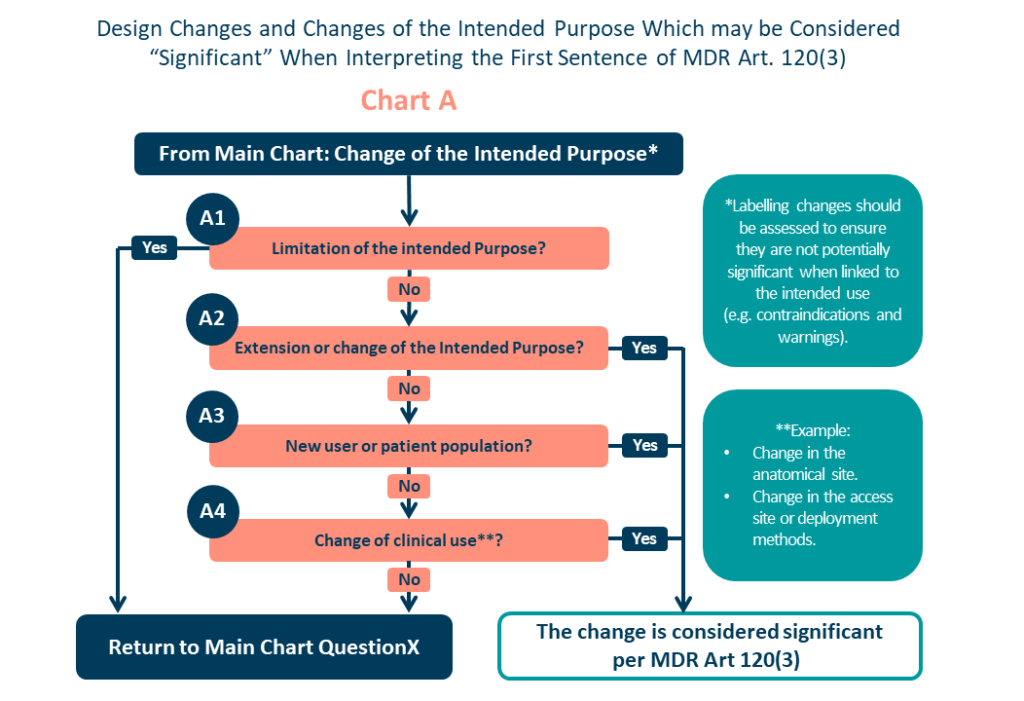
Your ‘Intended Purpose’ must always be the foundation for all decisions, including whether the item qualifies as a medical device. Correctly described, the ‘Intended Purpose’ will provide:
- Strategic alignment within your Clinical Development Plan (CDP) between your Business Development, Regulatory, Device Design & Development and Quality Assurance plans.
- Strategic alignment in your Design Process, especially in your Usability (ISO 62366).
- Whether the product being considered fits the definition of a “medical device” and if the regulation applies.
- The basis for classifying the future planned device into one of the four device classes, as required by Article 51.
- Core text needed for future labelling, instructions, promotional or sales materials, clinical evaluation, and technical documentation.
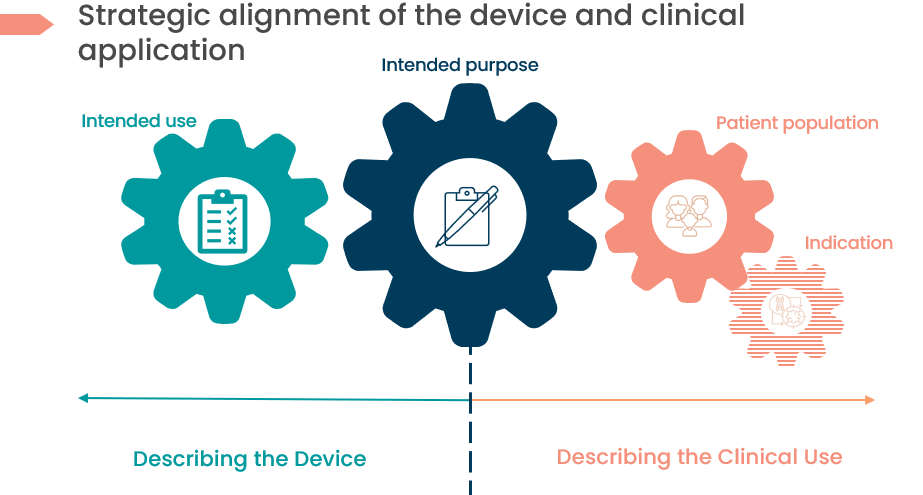
Solution 1: Linking back to your CDP and Business Development Plans
The link between Clinical Development Plan and Business Development Plans is really all thanks to the ‘‘Intended Purpose’’. The Clinical Development Plan in your MDR Technical File is commonly forgotten and neglected, resulting in non-conformance audit findings.
According to the EU MDR, the CDP’s purpose is:
“a clinical development plan indicating progression from exploratory investigations, such as first-in-man studies, feasibility, and pilot studies, to confirmatory investigations, such as pivotal clinical investigations, and a PMCF as referred to in Part B of this Annex with an indication of milestones and a description of potential acceptance criteria;”
“generate, through properly designed clinical investigations in accordance with the clinical development plan, any new or additional clinical data necessary to address outstanding issues; and analyse all relevant clinical data in order to reach conclusions about the safety and clinical performance of the device including its clinical benefits.”
Don’t be misled; this is no small nor obscure document. Specific clinical information exemplifies the importance of specifying your “Intended Purpose” and showing clinical performance, safety, and advantages. Additionally, it supports any future marketing claims you make to advance, promote, and advertise your product.
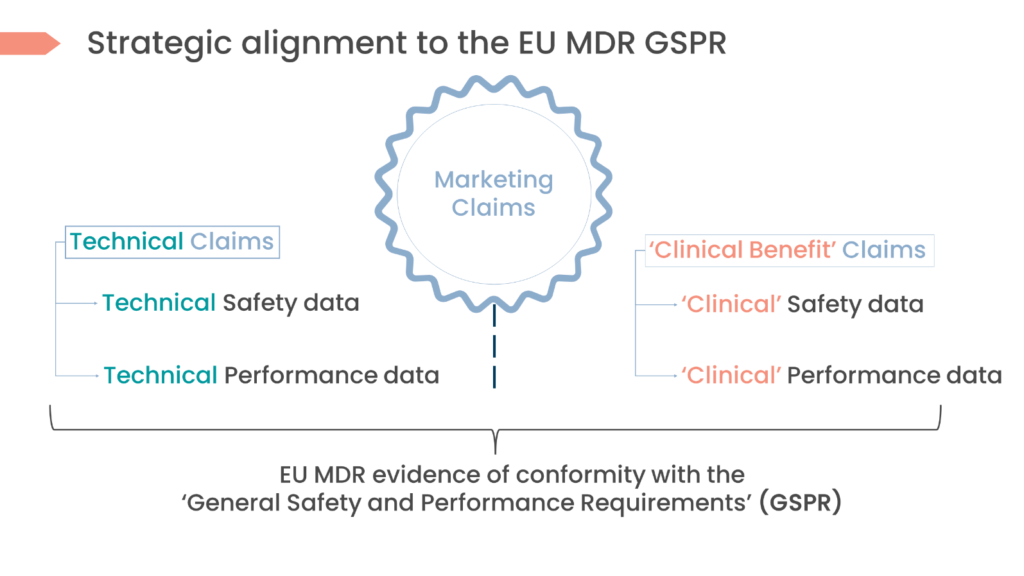
Solution 2: Really understand the key definitions needed for alignment
Avoid getting “Intended” or “Indications for” mixed up. The vocabulary of the regulations isn’t harmonised with the healthcare industry it serves and other regulatory jurisdictions outside of Europe, which is why it is so unclear.
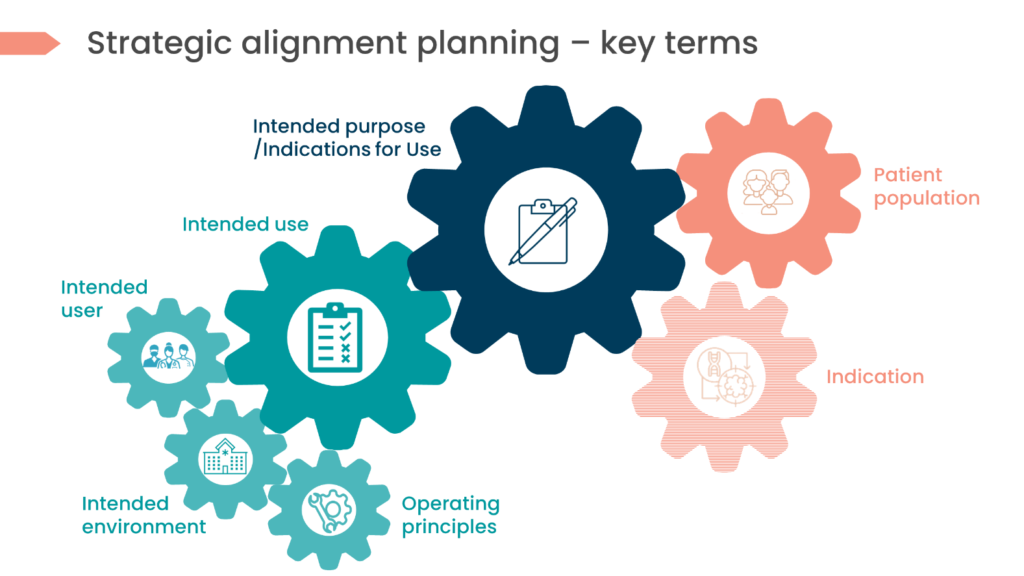
Yes, device terminology can be confusing, but we can avoid this by strategically planning ahead at the onset of development. We supply key definitions of terminology that need to be defined initially to ensure alignment in your medical device CDP and marketing strategies in the table and picture below, respectively.
Key Terms | Definition |
‘Intended Purpose’/ Indications for use | The use for which the device is intended according to the data supplied by the manufacturer on the labelling, in the instructions and/or in promotional material.Source: Article 1.2 (h) of the IVDD and Article 1.2 (g) of the MDD and MDR |
Intended use | INTENDED USE focuses on the medical purpose.Source: IEC 62366-1:2015Is the medical indication of the product, process, or service hence the medical procedure in which the medical device is used.Source: GHTF/SG1/N70:2011 Note: the MDR defines ‘‘Intended Purpose’’, but not ‘intended use’. |
Indication | Refers to the clinical condition that is to be diagnosed, prevented, monitored, treated, alleviated, compensated for, replaced, modified, or controlled by the medical device.Source: MDCG 2020-6 Note: not defined in Article 2 of EU MDR. |
Intended Users | Persons interacting with (i.e. operating or handling) the medical device.Source: IEC 62366-1:2015 Note: Not defined in Article 2 of EU MDR. |
Intended Patient Population | Patient population & medical conditions to be diagnosed, treated and/or monitored & other considerations, such as patient selection criteria, indications, contraindications, and warnings. |
Principles of Operation | Principles of operation of the device and its mode of action, scientifically demonstrated if necessary. |
Problems with the current terminology come in various shapes and forms.
Where devices do not directly achieve a positive impact on the patients, they may enable:
- A procedure to be undertaken.
- Therapy to be delivered.
- Allow another device to achieve its ‘Intended Purpose’.
- Devices used in so many procedures that it would not be practical or beneficial to define the ‘Intended Purpose’.
- Some procedures are generic and underpin the clinical use across patient groups and conditions.
- The definition of “indication” in MDCG 2020-6 makes clear that not all devices have indications: The clinical condition identified, avoided, monitored, treated, eased, compensated for, replaced, modified, or managed by the medical device referred to as the “indication” or “indication for use.” Contrast that with “‘Intended Purpose’/intended use,” which specifies how a thing works (X used in X procedure to do X). Although every device has a planned use or purpose, not every device has an indication. (E.g. medical devices with an ‘Intended Purpose’ of disinfection or sterilisation of other devices).
Solution 3: Design for ‘‘Intended Purpose’’
According to the MDR, “[medical devices] shall be developed and manufactured in such a way that they are fit for their intended function under normal conditions of usage” (Annex I, Chapter I, Paragraph 1). Manufacturers must first define the use specification to ensure their product is appropriate for the intended application(s).
The user interface specification (IEC 62366-1:2015, formerly known as the usability specification per IEC 62366:2007), which outlines the user interface requirements linked to usability, overlaps with the usability engineering process. When deciding what the needs are, the usage specification can offer direction. Although they start at the beginning of the product development process, the usage and user interface specifications are ongoing documents for many device manufacturers. As manufacturers gain more understanding of the product through usability engineering activities like known problems analysis and usability testing, the usage specification changes over time. To more effectively reduce use-related risk, we update the user interface definition as user interface designs change. We must complete these tasks to satisfy the MDR, and they also promote safer medical practices.
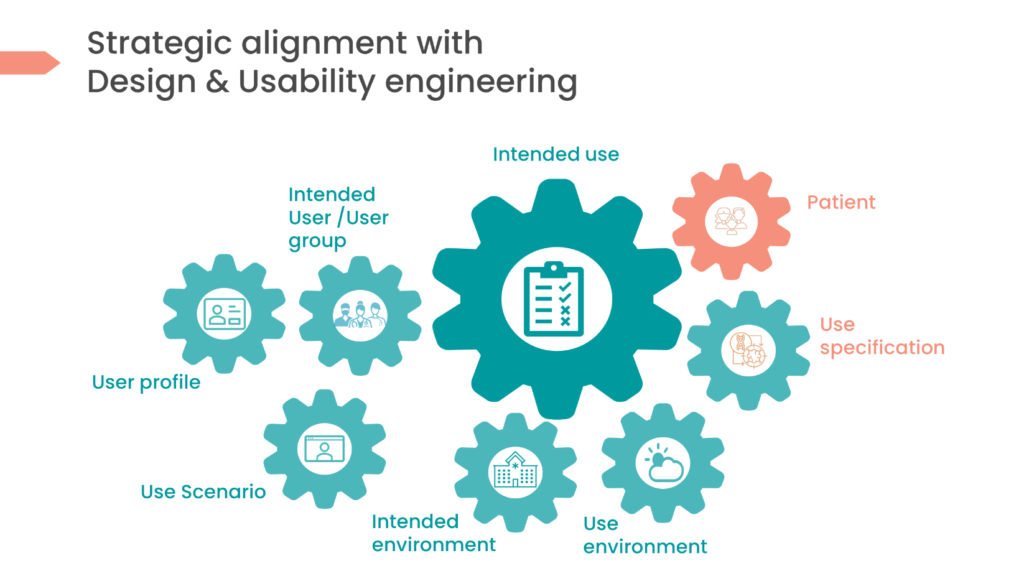
In conclusion, manufacturers can better comprehend and consider the device’s ‘Intended Purpose’ throughout development if the usability engineering process is applied. Devices properly built for the intended users will document the aspects related to the ‘Intended Purpose’ and take them into account throughout creation. To demonstrate MDR compliance, manufacturers must state their medical devices “Intended Use”. They can better comprehend and consider the device’s intended function throughout development if the usability engineering method is applied. Devices properly built for the intended users will result from correctly defining the main terms in usability (see table below) to align aspects of the ‘Intended Purpose’.
Overlapping Usability Terms | Definition |
1. User | The person interacting with (i.e. operating or handling) the MEDICAL DEVICE. Source: BS EN 62366-1:2015+A1:2020 |
2. User Group | A subset of USERS who are differentiated from other USERS by factors that are likely to influence their interactions with the MEDICAL DEVICE Source: BS EN 62366-1:2015+A1:2020 Note1 to entry: Attributes of USER GROUPS can include age, culture, expertise. |
3. Use Specification | APPLICATION SPECIFICATION – Summary of the important characteristics related to the context of use of the MEDICAL DEVICE. Source: BS EN 62366-1:2015+A1:2020 Note1 to entry: The intended medical indication, PATIENT population, part of the body or type of tissue interacted with, USER PROFILE, USE ENVIRONMENT, and operating principle are typical elements of the USE SPECIFICATION. Note2 to entry: The summary of the MEDICAL DEVICE USE SPECIFICATION is referred to by some authorities having jurisdiction as the ‘statement of intended use’. Note3 to entry: The USE SPECIFICATION is an input to determining the INTENDED USE of ISO 14971:2019 |
4. User Profile | Summary of the mental, physical & demographic traits of a USER GROUP, as well as characteristics, such as knowledge, skills and abilities, which can affect design decisions. Source: BS EN 62366-1:2015+A1:2020 |
5. Use Scenario | Specific sequence of TASKS performed by a specific USER in a specific USE ENVIRONMENT and any resulting response of the MEDICAL DEVICE. Source: BS EN 62366-1:2015+A1:2020 |
6. Patient | A living being (person) undergoing a medical, surgical, or dental procedure. Note1 to entry: A PATIENT can be a USER. Source: BS EN 62366-1:2015+A1:2020, IEC 60601-1:2005 & IEC 60601-1:2005/AMD1:2012, 3.76, modified – The phrase ‘or animal’ has been deleted from the definition and “USER” has been substituted for “operator” in the note to entry. |
7. Usability | Characteristic of the USER INTERFACE that facilitates use and thereby establishes EFFECTIVENESS, EFFICIENCY and USER satisfaction in the intended USE ENVIRONMENT. Source: BS EN 62366-1:2015+A1:2020 |
8. Use Environment | Actual conditions and setting in which USERS interact with the MEDICAL DEVICE. |
9. Use Specification/User Interface Specification | A collection of specifications that comprehensively and prospectively describe the USER INTERFACE of a MEDICAL DEVICE. Source: BS EN 62366-1:2015+A1:2020 |
CLIN-r+ Recommendations in summary
Firstly, hang in there! You are not the only manufacturer management team or medical device employee trying to survive this regulatory change. Be active in following Medical Device groups that help highlight new guidance and insights.
Deploy the key strategies to avoid having to submit a ‘Significant Change’ to your Notified body by deploying our three key strategies to make the ‘‘Intended Purpose’’ and its underpinning terms work for your medical device with the following solutions:
- Start your device planning with your CDP, bring in your Business Development Plan to ensure the key terms for ‘‘Intended Purpose’’ aligns. Then bring in all key stakeholders (Regulatory, Quality, Clinical, R&D and Marketing) to review the terms and share insights on what they need to align their processes and the impact on the Technical Document files.
- Train your regulatory, clinical affairs, marketing and R&D engineers to understand the key definitions needed for alignment with ‘‘Intended Purpose’’ for Medical Devices.
- Ensure the key terms drive your design process for ‘‘Intended Purpose’’ which relate to describing your device and its usability planning. Defining your devices ‘Intended use’, ‘Intended User’, ‘Intended Environment’ and ‘Operating principles’ to align with your ‘‘Intended Purpose’’ will drive your design process in the right direction.
We hope the information in this document helps you assess and manage the impact that ‘Intended Purpose’ will inevitably have on medical device development and marketing strategy under EU MDR. It can be a minefield, and having an external expert such as Clin-r+ can be an invaluable addition to your Medical Device armoury. Should you have any questions or need professional assistance, CLIN-r+ has a wealth of experience to call upon. Get in touch!

