Labels for Medical Devices
How to create a label as per EU MDR 2017/745
Sometimes viewed as “the finishing touch” or categorised as a “miscellaneous” task, the labels for medical devices are an indispensable part. Clin-r+ are here to help you understand labels and how to put them into action on your systems.
How do you create labels for Medical Devices?
This depends on the region where your products are being sold. Therefore, whilst the focus here is on the European Union market, manufacturers should note that label creation may vary if your products are being sold outside the EU.
The European Union’s Medical Device Regulation (MDR) defines “label” as:
“label” means any written, printed, or graphic information appearing either on the device itself, on the packaging of each unit, or on the packaging of multiple devices.
 The labelling process is used to identify medical devices and their manufacturers. It also serves the purpose of communicating essential information on safety, use, and performance.
The labelling process is used to identify medical devices and their manufacturers. It also serves the purpose of communicating essential information on safety, use, and performance.
Manufacturers must design the label with the user of the medical device in mind, both professionals, consumers and relevant third parties. Labels provide critical information for the user or patient, and are testament to their importance and why regulations exist, telling us exactly what needs to appear on them.
Labelling requirements under the new MDR
Under the EU MDR, device safety and clinical effectiveness data must be present on labels. General safety and performance requirements cover all requirements regarding the information supplied with the medical devices.
Two of the biggest challenges when trying to comply with the EU MDR labelling requirements are:
- Ensuring all necessary symbols and information are covered.
- The size of the label for medical devices. Since there are significantly more symbols and data required, how to fit it all on the label can pose problems.
 It is important to remember the following during label design: the medium, format, content, legibility, and location of the label and instructions must match the technical knowledge, experience, education, or training of the intended user(s).
It is important to remember the following during label design: the medium, format, content, legibility, and location of the label and instructions must match the technical knowledge, experience, education, or training of the intended user(s).
Therefore, it is essential to write the instructions in terms readily understood by the intended user. It can be useful to supplement the instructions with drawings and diagrams where appropriate.
It is worth mentioning that you have a choice in format (we provide an example in this article). Labels can be provided in human-readable format, but should also be supplemented by machine-readable information, for example for UDI.
Symbol vs no Symbol
As the old saying goes, a picture is worth a thousand words, so let’s see the impact that the use of symbols can have on your labels.
Below is a label without symbols. It contains all the required information, translated into the necessary languages for its intended market.

And here is the same label utilising symbols.
The use of symbols has enabled the information to be presented in a much more convenient, user-friendly, and tidy manner. A major advantage of using symbols is that one symbol can avoid translating information into multiple languages.
BSI Presentation on the standard of labels for medical devices
Having outlined the benefits of using symbols on labels for medical devices, it is worth noting that while you can create your own symbols, Clin-r+ highly recommends using international standards such as ISO 15223-1.
There is a new revision of the standard: ISO/DIS 15223-1:2020 Medical devices — symbols to be used with medical device labels, labelling and information to be supplied — Part 1: General requirements.
Since there are new MDR requirements, there are completely new symbols to cover them. If you would like to know more about the new symbols, acquire the ISO standard and guidance from CLIN-r+. Remember, you need to describe the symbols inside your instructions for use.
New revision of ISO 15223-1
To learn about the labels for medical devices standard from the BSI, download their presentation.
How to be prepared
Labelling challenges are common for medical device manufacturers when trying to be compliant with the MDR, even more so for those currently working without a labelling system fit for purpose.
A good first step in preparing your MDR labels is to reference which requirements are applicable to your medical device.
Define your requirements and then use the symbols to design your label. Keep in mind that due to regulations changing frequently, your label design should be as flexible as possible, as you will need to respond promptly to any changes.
Official Union Languages
As mandated by Article 10, manufacturers must ensure that the device is accompanied by the information set out in Section 23 of Annex I in an official Union language(s) determined by the Member State in which the device is made available to the user or patient. See the official union languages in the table below.
| Country | Language | Country | Language | |
| Austria | German | Latvia | Latvian | |
| Belgium | French, Dutch, and German | Liechtenstein | German | |
| Bulgaria | Bulgarian | Lithuania | Lithuanian | |
| Croatia | Croatian (and English) | Luxembourg | French or German or Luxembourgish | |
| Cyprus | Greek (and English) | Malta | English or Maltese | |
| Czech | Czech | The Netherlands | Dutch | |
| Denmark | Danish | Norway | Norwegian | |
| Estonia | Estonian | Poland | Polish | |
| Finland | Finnish and Swedish (or English) | Portugal | Portuguese | |
| France | French | Romania | Romanian | |
| Germany | German (and English if justified) | Slovakia | Slovak | |
| Greece | Greek | Slovenia | Slovene | |
| Hungary | Hungarian | Spain | Spanish | |
| Iceland | Icelandic | Sweden | Swedish | |
| Ireland | English (or English and Irish) | Switzerland | German, French, and Italian, EPU | |
| Italy | Italian | Turkey | Turkish |
When do the new EU Medical Device Regulations need to be met?
The implementation of the new EU Medical Device Regulation had an original transition period of three years, meaning that articles of regulation would have been enforced from 26th May 2020. The COVID-19 epidemic prompted a new deadline of 26 May 2021.
Class I devices, unless still benefiting from a transition period (Software or Reusable Surgical Instruments), should have had their labels updated immediately from 26th May 2021.
Any products that remain under MDD can continue with their current label. Nevertheless, the moment they transition to MDR, they will require the label to be updated.
Entry into force of Article 27(4)
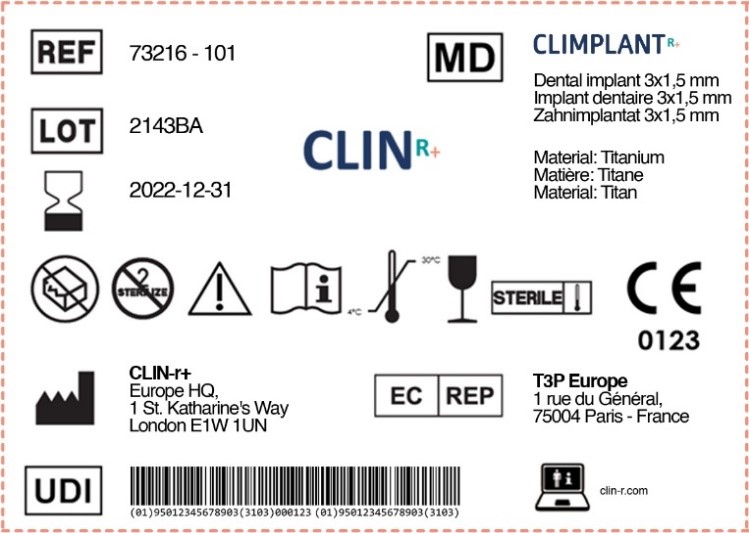
Manufacturers must place UDI carriers on the labels for medical devices and on all higher levels of packaging. Shipping containers aren’t considered high-level packaging.
| Class I | Class IIa & IIb | Class III | |
| UDI on device labels | 26th May 2025 | 26th May 2023 | 26th May 2021 |
| Reusable devices | 26TH May 2027 | 26th May 2025 | 26th May 2023 |
Innovations by MDR 2017/745
Manufacturers of medical devices are subject to a number of new requirements as a result of the EU Medical Device Directive. The new requirements are as follows:
- The scope of application also extends to non-medical products (e.g., contact lenses, devices for liposuction).
- Each medical device must bear a unique identification number (UDI).
- Manufacturers must register themselves and their products via the Europe-wide EUDAMED database and submit their data.
- Higher requirements are placed on technical documentation, especially in connection with the required risk management.
- Clinical trials and evaluations are being tightened and products are being monitored even after their market launch.
- Responsible persons who have qualified expertise on the manufacturing of medical devices must be appointed.
- For high-risk medical devices, an additional control procedure (called ‘scrutiny procedure’) for conformity assessment by a panel of experts is planned.
- Notified bodies are more strictly regulated, so that a renewed selection and inspection may be necessary.
What are the new elements on the labels for medical devices?
One fundamental change to the labels for medical devices is that manufacturers must state that their product is a Medical Device.
If Medical Devices are being sold in many countries, it is now required that all the information on the label be translated into the languages required by the market. Alternatively, manufacturers can use the MD symbols to avoid written translations.
Another significant change to the labels is the inclusion of the UDI code. You can include your product’s UDI code today if your product is compliant to EU MDR.
An additional new element for special devices is the need to clarify the MD’s status. If, for example, the device is only intended for clinical investigation, then the labels must contain the words “exclusively for clinical investigation”. This is also the case for any devices that are “custom made”.
Another new element to the labels is the mention of CMR. This is required for any products containing more than 0.01% w/w CMR substance.
The next section provides the list of requirements coming from Annex I chapter III.
Medical Devices Labelling Checklist for EU MDR Compliance:
The EU MDR transition can be challenging, and it is essential that manufacturers conduct the labelling criteria with care, maintaining high levels of quality and safety for enforcement.
Manufacturers must consider the current labelling standards prior to implementation. Labelling errors could lead to Product Recalls and prove costly. This only reinforces our statement at the start of this document that the label is a crucial part of the device. Errors found on the label are essentially errors on the device, and it is not uncommon that manufacturers recall products due to incorrect information on the label.
Therefore, it is important to carefully plan medical product labels that comply with EU MDR labelling criteria. Use the following checklist as a reference.
Medical Device Labelling Requirements as per EU MDR:
- If there is no expiration date, manufacturers should include the device’s name, trade name, and manufacturing date.
- On all product labels being delivered into the EU, a standardised symbol/logo/icon must indicate the product is a medical device.
- The label must provide the packaging’s contents and intended use for a customer to recognise.
- If the manufacturer is outside the EU, they must prominently display the approved licensed EU representative details on the label.
- The label should state the time limit (year and month) for using or implanting the product safely.
- Manufacturers must list on the label the warnings, instructions, precautions, or contraindications for the immediate attention of the user, while in use.
- The Unique Device Identification (UDI) carrier should be present on each label. Unique identifying “part numbers” issued by a neutral party and allocated to finished products, product packaging with UDI logo, UDI on the product itself (direct part marking) for certain products are all necessary components of a UDI. You must register UDIs in the EUDAMED database.
- Web address and eIFU (electronic Instructions for Use).
- The UDI symbol’s composition must include the Device Identifier (DI) and Production Identifier (PI).
- Machine-readable information can be on the labels, which must be in a human-readable format.
- For devices with absorbent materials, the labels must provide composition and quantitative details on the key constituent(s).
- Product name and lot or serial number.
- All details needed for a user to identify the device, the contents of its packaging, and the intended purpose of the device.
- Contact details of the manufacturer (e.g., name and address).
- In the case of non-EU based manufacturers, the name and address of their authorised representative.
- Where relevant, information per Section 10.4.5. of the MDR.
The UDI carrier referred to in Part C of Annex VII of the MDR
- Any special storage or handling conditions.
- A clear indication of the time limit for using the device safely, or where this is not applicable, the date of manufacture.
- When the supplied product is in a sterile condition, an indication of its sterile state and method of sterilisation.
- If the product is for single use.
- If the device is custom-made, or intended for clinical investigation only.
- Other warnings and precautions the user should immediately be aware of.
Recognising and enforcing the EU MDR labelling criteria on the previous page is indeed a challenging part of making your medical device label EU MDR compliant. However, failure to follow proper protocols can lead to regulatory enforcement issues and expensive product recalls. Hence the importance that manufacturers are aware of the latest MDR labelling standards in the EU.
What about the design of your device labels? Are they effective and flexible? Are they compliant? For a direct assessment, contact a Regulatory Device Labelling specialist, such as CLIN-r+, and keep your labels up to date.
Manufacturer or Legal Manufacturer?
When referring to a Legal Manufacturer of a Device, this doesn’t always mean that said body has any manufacturing processes, but instead to the body taking responsibility for this product in the European Market. We can find this status of a device on the label of the Medical Device.
There is often confusion regarding both Own Brand Labelling (OBL) and Original Equipment Manufacturer (OEM). The EU MDR no longer authorises this economic model, and whilst you can still ask someone to manufacture a product for you, we will consider them the suppliers, not the Legal Manufacturers.
 It is critical that you elect your status. If you are the product’s Legal Manufacturer, you must have your name behind the Black Plant Symbol. For example, any manufacturer based in Europe, whose Medical Device is produced in China, will need to ensure their name appears behind the Black Plant Symbol if they want to be the legal manufacturer of that product.
It is critical that you elect your status. If you are the product’s Legal Manufacturer, you must have your name behind the Black Plant Symbol. For example, any manufacturer based in Europe, whose Medical Device is produced in China, will need to ensure their name appears behind the Black Plant Symbol if they want to be the legal manufacturer of that product.
However, if you wish to follow where you elect to have your Supplier as the Legal Manufacturer, but using your trademark, the supplier will have their name behind the Black Plant Symbol. In this instance, we would find your name behind the Importer or Distributor Symbol.
Importers and Distributors
As mandated in Article 13, importers must list their name, registered trade name or registered trademark, registered place of business, and contact information on the device, its packaging, or in a document accompanying the device, so that their location can be determined. They must ensure that any additional labels do not obscure information on the manufacturer’s label.
While labels for medical devices do not have to include the distributor’s name, distributors do have obligations. As stated in Article 14, distributors must ensure the device is stored and transported in accordance with the manufacturer’s specifications while under their control.
Relabelling and Packaging Changes
There are instances where obligations usually applied to manufacturers will apply to importers and distributors. Article 16 mandates that a distributor or importer who performs any of the activities listed in paragraph 2 points (a) and (b) must indicate the activity performed on the device or, if that is not possible, on its packaging or in a document accompanying the device, along with its name, registered trade name or registered trademark, registered place of business, and contact information, so that its location can be determined.
Levels of labelling
It is possible to have multiple labels on different levels of a product, and it is important to understand at which level you are. Below, we will focus on the different levels of labelling and packaging.
Primary label
Usually found in the prominent position at the top or front of the product, the primary label is normally decorative, eye-catching, showing the company logo and brand colours. The primary label only includes the most important pieces of the product information, such as product name, contents, company name, and a slogan or tagline.
The primary label is to grab a potential customer’s attention and convey the brand identity of the product.
If the primary label is too small to contain all the information listed above (for example the UDI), you are permitted to transfer some information to the secondary label.
Secondary label
 A secondary label is generally placed in a less prominent position at the bottom, back, or side of a product. On the secondary label, you can find more specific product information, such as its ingredients and nutritional values, health and safety warnings, instructions for use, manufacturer or supplier details, contact information, detailed tracking and product information in a barcode format.
A secondary label is generally placed in a less prominent position at the bottom, back, or side of a product. On the secondary label, you can find more specific product information, such as its ingredients and nutritional values, health and safety warnings, instructions for use, manufacturer or supplier details, contact information, detailed tracking and product information in a barcode format.
The secondary label aims to provide potential customers with specific and more detailed information about the product they are considering purchasing. By specific, we refer to information specific to a product line, an individual product, or bespoke products with information unique to a single item.
Levels of packaging
Primary packaging
The primary packaging, also referred to as consumer or retail packaging, is the packaging that comes into direct contact with the product itself. Primary packaging is there principally to protect and preserve, but also to inform the consumer.
Secondary packaging
 The main purpose of the secondary packaging is branding, display and logistics. Secondary packaging includes packaging purposely made to display multiple product units for sale, which speeds restocking from storeroom to shelf. This packaging includes retail-ready packaging (RRP), shelf-ready packaging (SRP) or counter-top display units (CDUs).
The main purpose of the secondary packaging is branding, display and logistics. Secondary packaging includes packaging purposely made to display multiple product units for sale, which speeds restocking from storeroom to shelf. This packaging includes retail-ready packaging (RRP), shelf-ready packaging (SRP) or counter-top display units (CDUs).
In addition to these functions, secondary packaging protects and collates individual units during storage. It is often used by the beverage, food, and cosmetic sectors to display primary packs on shelves, and is sometimes referred to as grouped or display packaging.
Tertiary packaging
The purpose of the tertiary packaging is to facilitate the protection, handling, and transportation of a series of sales units (contained within secondary packaging), so that they can group everything into unit loads during transit. Tertiary packaging usually does not come into contact with the consumer.
CLIN-r+ top tips on being compliant:
We hope this document has not only highlighted the importance of the product labels for medical devices, but also provides you with a valuable framework to create/update your own labels.
Should you need professional assistance, CLIN-r+ have a wealth of experience in both the creation of labels and guidance on existing label design. Get in touch!