CER, SSCP and PSUR
What's the difference between CER, SSCP and PSUR?
All medical devices on the European market must be continuously monitored, without exception, for user safety and efficiency. EU MDR (2017/745) requires clinical evaluation and post market surveillance to ensure the safety of medical devices throughout their lifetime.
EU MDR and EU IVDR require a Periodic Safety Update Report (PSUR) and Summary of Safety and Clinical Performance (SSCP). They left many manufacturers unsure which, if any, of these documents are required and when. The clinical evaluation report (CER) contains all PSUR and SSCP information. So, what is the purpose of each report? How do they differ? Do you need a PSUR or SSCP if you have a CER?
Read on for a break-down of the aspiration and content of each report, and why both reports are required under the EU MDR.
Some of your documents will be made accessible to Third parties via Eudamed
Your NB will upload PSURs and SSCPs onto EUDAMED, which may surprise some manufacturers. Please see the flow of information and to whom it should go in the diagram below.
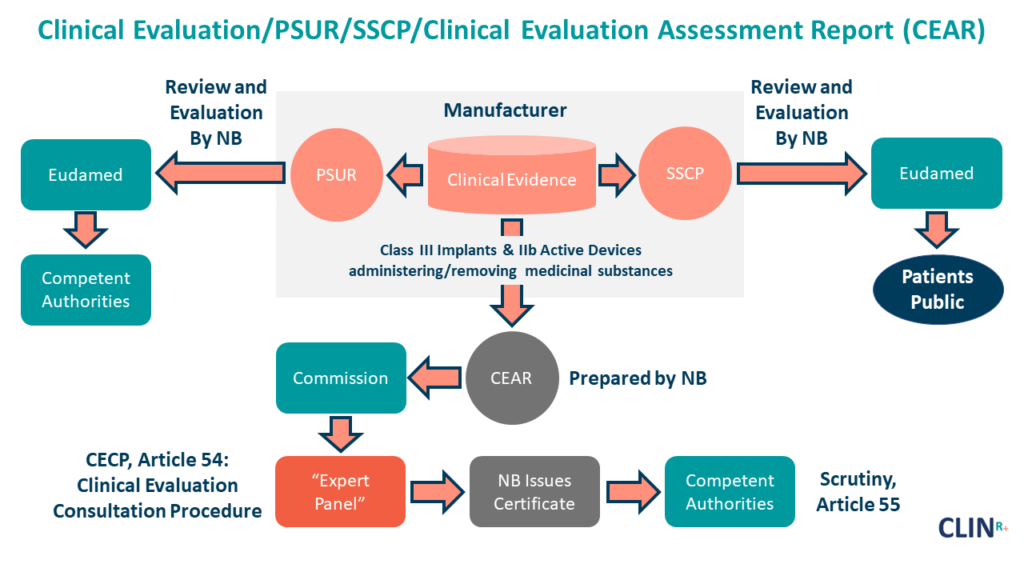
High-risk devices must submit their PSURs via an “electronic system” – essentially EUDAMED. Consequently, Class III/Class D device PSURs, SSCP and the NB evaluation are made available to competent authorities through EUDAMED. Once approved, your SSCP will also be made available to the public.
Going forward, the SSCP must be reviewed regularly, and updated when necessary, on EUDAMED. The aim of which is to ensure that clinical and safety information is accurate and complete. This is distinct from the PMCF evaluation report and PSUR, which are updated at least once a year.
Clinical Evaluation Report (CER) (MDR) (Note PER in IVDR)
The Clinical Evaluation Report (CER), an evaluation and conclusion of the clinical data collected for your device, is a crucial piece of the Technical Document for conformity assessment and subsequent CE marking in Europe.
The CER is designed to demonstrate the safety and efficiency of the medical device, whilst delivering on clinical performance. Moreover, a Clinical Evaluation Report documents both the assessment and analysis of clinical data collected, with the purpose of verifying the clinical safety and effectiveness of the device.
The report consists of a clinical investigation of the device itself and/or existing clinical studies for comparable devices.
Article 61 of the MDR:
 “A clinical evaluation shall follow a defined and methodologically sound procedure based on the following:
“A clinical evaluation shall follow a defined and methodologically sound procedure based on the following:
- Critical evaluation of the relevant scientific literature currently available relating to the safety, performance, design characteristics, and intended purpose of the device.
- A critical evaluation of the results of all available clinical investigations.
- A consideration of currently available alternative treatments options for that purpose, if any.”
Considering the vast array of medical devices, composing a definitive list of what should be included in the CER is difficult. Higher risk devices pose a greater potential threat to a patient’s health if they malfunction. Therefore, they require a more detailed CER than lower-risk devices.
Although no two CERs are the same, they must all contain certain elements:

- Device information, such as intended use, medical device design, manufacturer name, regulatory history, and any other relevant data.
- Technical device description, such as intended use and target population, classification, therapeutic or diagnostic claims, clinical background, etc.
- Any identified clinically equivalent device and a justification of its equivalence.
- Existing clinical investigation and data.
- Summary of clinical data and review, including documented systematic literature searches and reviews.
- Data appraisal methodology and parameters.
- Conclusions about safety, performance, and conformity, as well as benefit-risk determination.
PSURs are well-known in the pharmaceutical world, but they’re a new requirement in the medical device industry.
Due to a growth in unmonitored medical devices, the medical device sector anticipated the EU MDR will focus more on post-market surveillance data systems. The MDR did not disappoint, emphasising device safety over its lifetime. Post market surveillance is a critical part of a medical device manufacturer’s obligations and regulation.
Article 86 of the MDR mandates the Periodic Safety Update Report.
“Throughout the lifetime of the device concerned, the PSUR shall set out:
- The conclusions of the benefit-risk determination.
- The main findings of the PMCF.
- The volume of sales of the device, and an estimate of the size and other characteristics of the population using the device, and, where practicable, the usage frequency of the device.”
It is imperative that the report be updated continuously throughout the lifetime of the device, from the moment it is put on the market. The Periodic Safety Update Report is an integral part of the technical documentation submitted to the notified body during the conformity assessment.
Not a repetition of the PMSR, the PSUR is a distinct document underlining the safety of the medical device in question. Ordinarily, it would include the following elements:
- Conclusions on benefit-risk analysis.
- Findings from the post-market clinical follow-up.
- Sales volume and usage frequency of the device.
- Vigilance data.
- Descriptions and rationales for any preventive and/or corrective actions made.
- Results and conclusions on the post-market surveillance data.
So, what are the differences between the CER and PSUR?
While the two reports share some common elements, their purposes are distinct.
The Clinical Evaluation Report demonstrates safety and efficiency for a medical device’s clinical use. It interprets all the clinical data found on the device both before and after product launch.
PSUR is only concerned with demonstrating the safety and performance of the post-market data. It helps manufacturers demonstrate to NBs that their post market surveillance program uses market data to ensure device safety, efficacy, and performance. It proves the effectiveness of a proactive post market surveillance system.
So, what are the differences between the SSCP and PSUR?
MDR 2017/745 requires robust post market follow up for implantable and class III medical devices, for which the SSCP is crucial. The SSCP strives to provide healthcare workers and patients with updated clinical data and information about the medical device’s safety and clinical performance.
IVDR Article 29 of the European In Vitro Diagnostics Regulation 2017/746 (EU IVDR) outlines a requirement similar to the SSCP: the Summary of Safety and Performance (SSP) for Class C&D. It replaces ‘clinical’ with ‘performance’ evaluation, and requires metrological traceability of assigned values. Considering the pressure on commission expert groups to develop guidance documents, manufacturers can use MDCG 2019-9 as a model for SSP guidance. IVDR manufacturers may wish to look ahead to prepare.

The SSCP should have two parts. First for healthcare professionals, and the second for patients. The challenge here is a writeup that targets both audiences. Such writing requires strong technical writing skills and the ability to simplify complex scientific information.
There are 9 sections that need to be addressed in the SSCP:
- The identification of the device and the manufacturer, including the Basic UDI-DI and, if already issued, the SRN (single registration number).
- The intended purpose of the device, any indications, contraindications, and target populations.
- A description of the device, including a reference to previous generations of variants if such exist. And a description of the differences, as well as, where relevant, a description of any accessories, other devices and products intended to be used in combination with the device.
- Information on any residual risks and any undesirable effects, warnings and precautions.
- The summary of clinical evaluation as referred to in Annex XIV, and relevant information on post market clinical follow-up.
- Possible diagnostic or therapeutic alternatives.
- Suggested profile and training for users.
- Reference to any harmonised standards and CS applied.
- Revision history.
It is important to remember that the SSCP provides information in abundance. MDCG 2019-9 clearly states that SSCP does not:
- Give general advice on the diagnosis or treatment of particular medical conditions.
- Replace the instructions for use (IFU) as the main document provided to ensure the safe use of a particular device.
- Replace the mandatory information on implant cards or in any other mandatory documents.
Below, you will find a summary of the elements included in each report, and below that are the all-important guidelines for keeping each report updated.
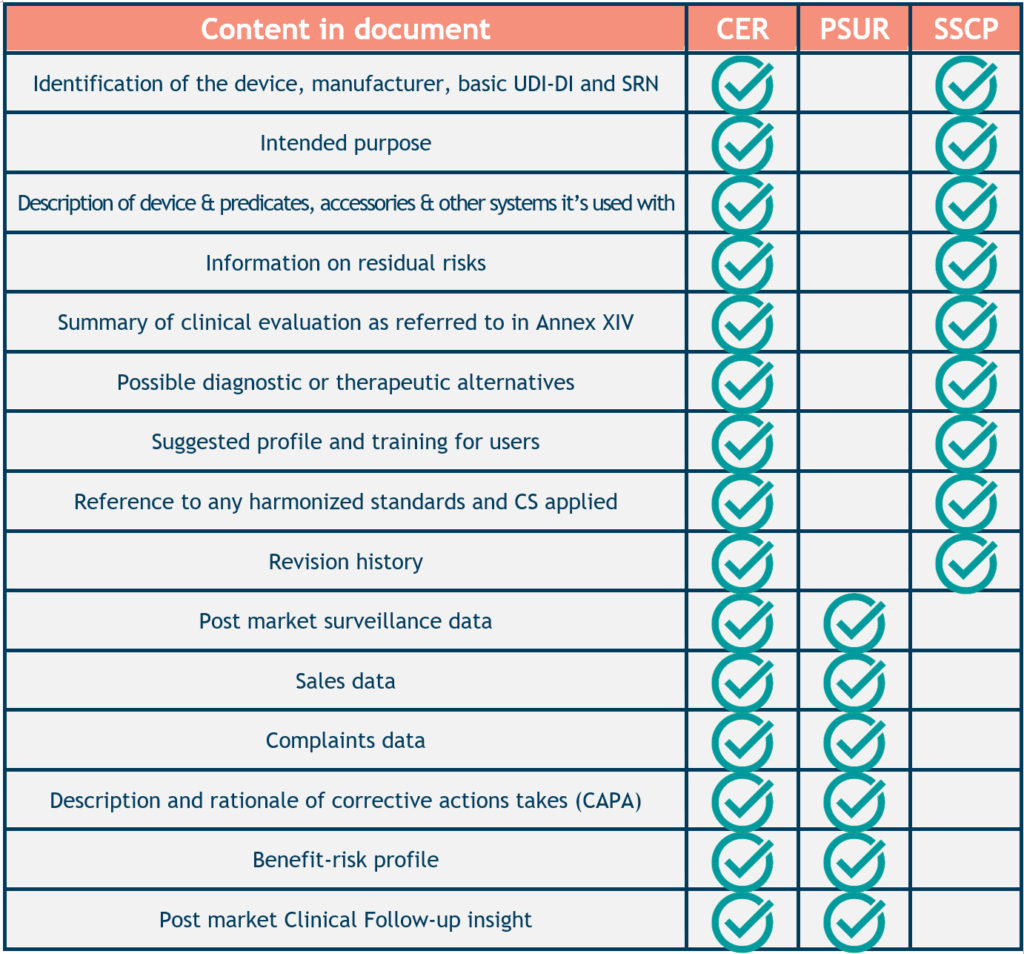
CER Updates: You must update Class I when needed, while Class II requires updating every 2 to 5 years. You must update Class III must annually, but Class A & B devices as needed. And lastly, you must update Class C & D devices at least every year. All classes need to be updated due to new information changing the benefit to risk or if new risks arise.
PSUR Updates: The PSUR is part of a device’s technical documentation. You must update this throughout the device’s life cycle.
SSCP Update: The SSCP is part of a device’s technical documentation. You need to update it to reflect PER or CER changes that affect the benefit-to-risk of the device.
CLIN-r+ Recommendation:
Manufacturers should consider these reports as a work stream of data that requires reporting in several ways. The diagram below demonstrates the flow of data required under MDR and IVDR. This can transform an organisation’s IT infrastructure, work roles and interdepartmental collaboration.
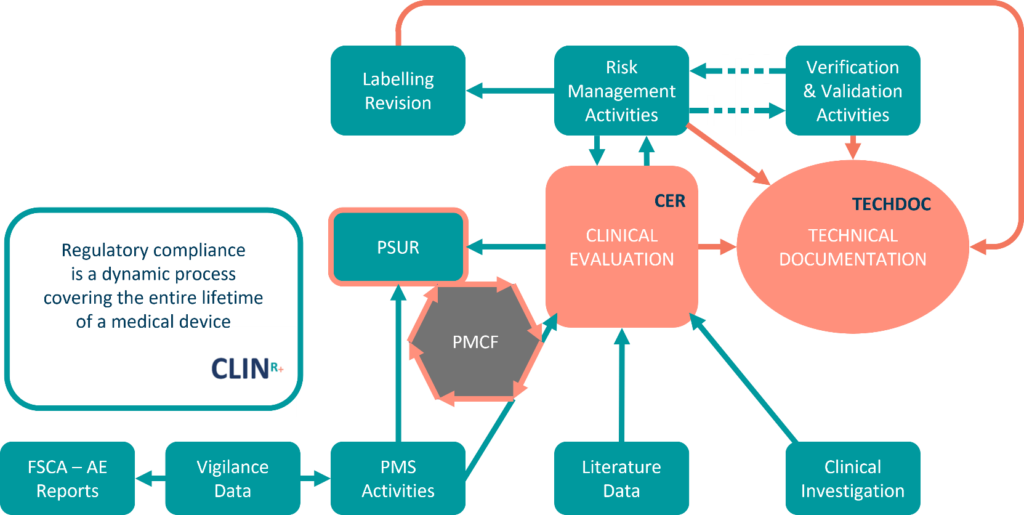
EU MDR emphasises efficient clinical evaluation and post-market surveillance systems, putting pressure on medical device manufacturers. Pre- and post-launch work will focus on how, where, and when to adapt internal infrastructure to streamline work. This is largely due to NB increased vigilance in their reviews of QMS systems, engineering reports, and ensuring compliance with clinical and post-market requirements.
These requirements can be challenging. The CLIN-r+ team can help plan your technical documents, formulate workflows (QMS SOP’s), and work with your team to streamline this change. Initially, where there may not be enough resources, count on CLIN-r+ to form part of your organisation to ensure the workflow progresses and that you can meet the full MDR/IVDR transition on time. Interested? Get in touch!



